…article continued:
Results
The DNA ConneXions Oral Panel report provides a general description, symptoms of infection, and CDC recommended treatment options for each organism detected. A Total Risk Factor is calculated for each organism which considers the concentration of the organism present (Measured Risk Factor) and the characteristics of that organism (Pathogen Risk Factor). Pathogen Risk Factors are determined based on the overall characteristics of the organism: its overall pathogenicity and ability to cause disease, resistance to treatment, illness causing properties, links to systemic diseases, etc. The Measured Risk Factor is based on the visualized intensity of the amplification product, which has a direct linear correlation to the amount of the organismal starting material present in the sample.
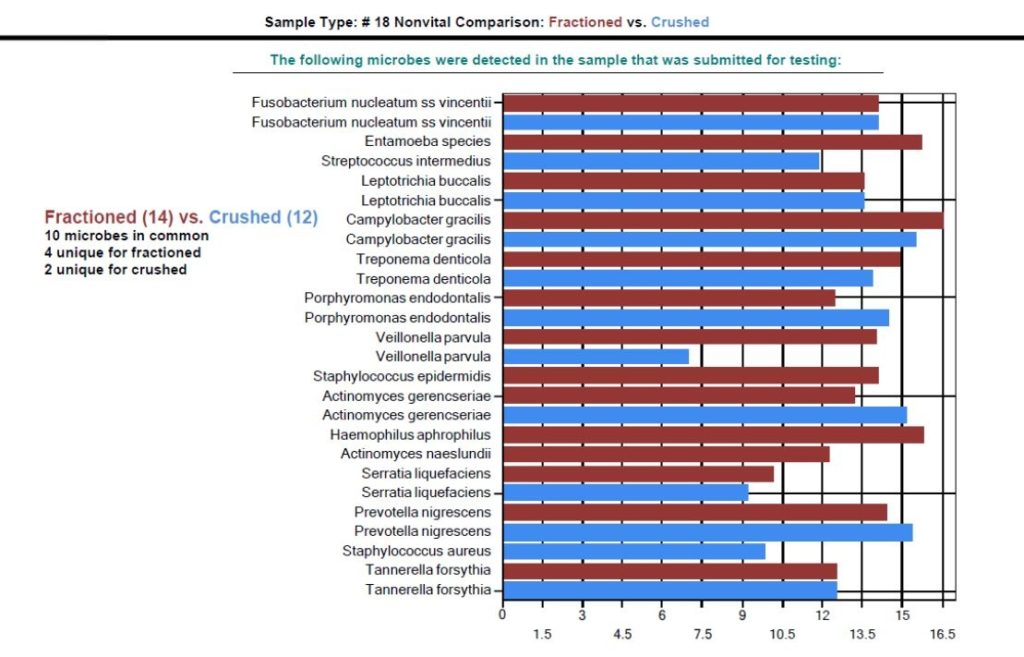
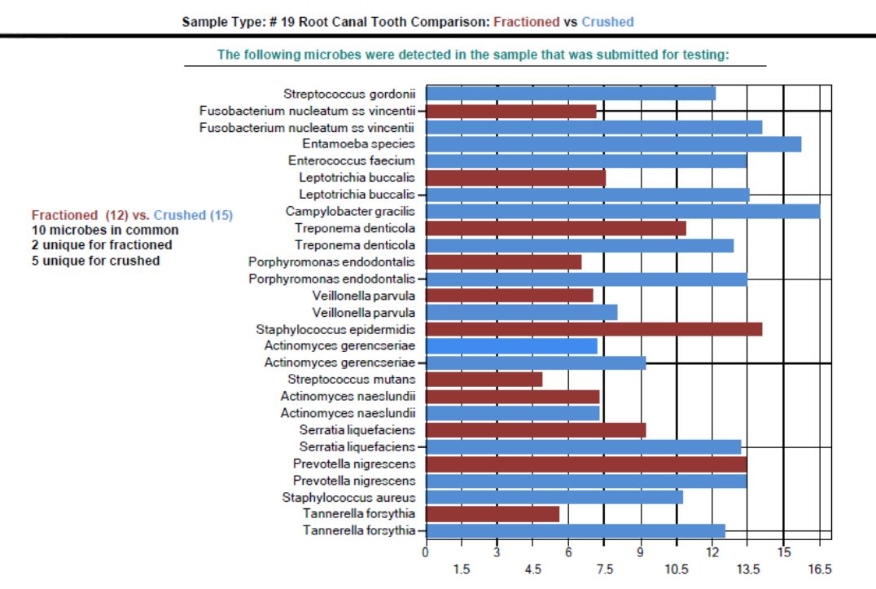
Utilizing the proprietary DNA ConneXions Alpha-5 software, the combination of both the Measured Risk Factor and the Pathogen Risk Factor yields the Total Risk Factor of each organism detected. Figure 3 and Figure 4 graphically display both the whole and ground microbial flora detected with the DNA ConneXions Oral Panel, of #18 Nonvital Tooth and #19 Root Canal Tooth, respectively. Whole sample results are shown in red and ground sample results are shown in blue. The horizontal scale represents the Total Risk Factor for each microbe, which is based on the quantity of the organisms detected combined with the biological attributes of the organism.
(Fractioned and Ground Comparison)
(Fractioned and Ground Comparison)
While there are some differences seen between the amounts and identity of the organisms detected for each sampling type, the fractioned vs. ground results are well within the expected range we typically see for the DNA ConneXions Oral Panel, which is between 12-17 microbes detected. The slight differences could be due to the manipulation of the sample (ex. fractioning and/or grinding) and may have had an impact on the PCR results. Based on the number and concentration of microbes detected for the above sampling types, we determined that these variances are not statistically significant; and in general there was an overall consistency seen within the fractioned and ground sampling types, and the typically received whole tooth sampling type.

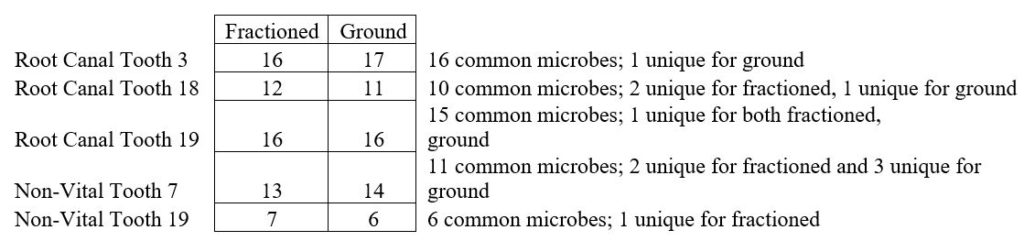
Repeated and various samples from additional participants yielded consistent results with respect to total number of microbes detected, microbes in common between whole and crushed samples, and number of amplification products typically seen on the DNA Connexions Oral Panel (Table 1).
Conclusions
We have included research on some of the most common oral microbes that we commonly detect in utilizing the DNA ConneXions Oral Panel. The reports produced for both site 18 (nonvital tooth), 16 amplification products, and site 19 (root canal tooth), 17 amplification products, are typical Oral Panel reports. Our average number of amplification products spanning several thousand reports is between 12-17, with the highest ever at 52. As the crushed and fractioned samples yielded the same average number of results as previous whole tooth samples, it leads us to conclude that these sampling methods are equivalent and equally effective in the DNA extraction techniques utilized at DNA ConneXions.
We do not instruct dental practitioners to fraction or otherwise prepare their submitted tooth samples. Typically, we do not receive fractioned samples, unless it naturally occurs during surgical removal. It was possible that the fractionation and/or grinding of the tooth would yield more prolific results. However, based on the average number of amplification products achieved with whole, fractioned and ground samples, this hypothesis was negated. Had the fractioned and/or ground sample yielded far more amplification products it would have led us to remodel our theory on the aliquot protocol in place at DNA ConneXions. Rather, the ‘unremarkable’ and ‘average’ results suggest that the porosity of teeth allows for the transfer of the microbial flora present during the aliquot protocol, therefore, we were able to definitively show that it is not necessary to fraction or otherwise grind the sample in order to achieve an accurate representation of the microbial flora present. The importance of this type of DNA testing is evident, enabling us to assist both doctors and dentists by identifying potential pathogens that are linked to common diseases, using either sampling method. This insight can provide valuable information to aid in the implementation of appropriate interventions for the patient.
Special acknowledgement to Kelly J. Blodgett, DMD, NMD, IBDM of Blodgett Dental Care, Portland, Oregon. (503) 713-6980. info@bdcpdx.com.
References
- Kane SF. The effects of oral health on systemic health. General Dentistry 2017; 411:35
- The World Health Organization. Newsroom. March 25, 2020. https://www.who.int/news-room/fact-sheets/detail/oral-health
- Peres MA, et al. Oral diseases: a global public health challenge. The Lancet. 2019; Vol 394
- Chen X, et al. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020; 9 (7): 569.
- Centers for Disease Control and Prevention. Periodontal Disease. Available at: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html. Accessed October 17, 2020
- Krishnan K, et al. A practical guide to the oral microbiome and its relation to health and disease. Oral Diseases. 2016; 23 (3)
- Kary B. Mullis Facts. The Nobel Prize. 1993. https://www.nobelprize.org/prizes/chemistry/1993/mullis/facts/ . Accessed October 17, 2020
- Maheaswari R, et al. Polymerase Chain Reaction: A Molecular Diagnostic Tool in Periodontology. J Indian Soc Periodontol. 2016 Mar-Apr; 20(2): 128–135.
- Fiorillo L, et al, Porphyromonas gingivalis, Periodontal and Systemic Implications: A Systematic Review. Dent. J. 2019; 7(4): 114
- Dineshkumar T. Role of Forensic Odontologist in Dentistry. Oral and Maxillofacial Pathology Journal. 2017;8(2):136-138
- Patil SH, et al. Polymerase Chain Reaction: An Innovative Tool in Periodontal Diagnosis. World J Dent. 2013;4(1):60-66
- Levy TE. Oral Pathogens: A Common Cause of Chronic Disease. JOM. 2016; 31(1) 7-18
- Graves DT, et al. The Oral Microbiota Is Modified by Systemic Diseases. J Dent Res. 2019; 98 (2):148-156
- Kreth J, et al. Bacterial and Host Interactions of Oral Streptococci. DNA Cell Biol. 2009; 28(8): 397-403
- Nakano K, et al. Detection of Cariogenic Streptococcus mutans in Extirpated Heart Valve and Atheromatous Plaque Specimens. Journal of Clinical Microbiology. 2006; 3313-3317
- Li J, et al. Actinomyces and Alimentary Tract Diseases: A Review of Its Biological Functions and Pathology. Biomed Res Int. 2018; 2018: 3820215.
- Liu J, et al. Analysis of Oral Microbiota Revealed High Abundance of Prevotella Intermedia in Gout Patients. Cell Physiol Biochem. 2018; 49:1804–1812
- Dominy SS, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Science Advances. 2019; 5(1): eaau3333.
- Jolivet-Gougeon A. Capnocytophaga species. Antimicrobe. Available at: http://www.antimicrobe.org/b92.asp#r57. Accessed October 25, 2020
- Yang K, et al. Corynebacteria as a cause of pulmonary infection: a case series and literature review. Pneumonia. 2018; 10 (10)
- Han YW. Fusobacterium nucleatum ssp: a commensal-turned pathogen. Curr Opin Microbiol. 2015 Feb; 0: 141–147.
- Tabassum S, et al. Failure of endodontic treatment: The usual suspects. Eur J Dent. 2016; 10(1): 144–147.
- Alghamdi F, et al. Influence of Treponema denticola on apical periodontitis due to infection of endodontal origin. International of Applied Dental Sciences. 2019; 5(3): 172-175
- Rocas IN, Siqueira Jr JF. Frequency and levels of candidate endodontic pathogens in acute apical abscesses as compared to asymptomatic apical periodontitis. Plos One. January 2, 2018.
- Alghamdi F et al. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus. 2020 Mar; 12(3): e7257.
- Tiwari S, et al. Detection of Red complex bacteria, P. gingivalis, T. denticola and T. forsythia in infected root canals and their association with clinical signs and symptoms. J Family Medicine and Primary Care.2020; 9(4):1915-1950
- Lačević et al. Correlation of Periodontal Pathogens in Concurrent Endodontic-Periodontal Diseases. Oral Biology and Dentistry 2015. Oral Biology and Dentistry. 2015.
Dr. Leslie J. Douglas completed her undergraduate studies in biology at the University of Hawaii at Hilo (UHH) before attending the University of Hawaii at Manoa (UHM), Department of Genetics and Molecular Biology. Currently, she is the principal investigator and laboratory manager of DNA ConneXions, a Colorado-based company focusing on bacterial, viral, fungal, and parasitic molecular-based detection assays. Her main focus is the research and development of a PCR-based Lyme test inclusive of Borrelia burgdorferi and a number of prevalent tick-borne disease co-infections, as well as the ongoing development of various molecular-based assays. Dr. Douglas’s research and patient demographics is yielding invaluable data to better understand the relationships between Lyme and other chronic conditions.
Blanche D. Grube graduated from Queens College, CUNY and received her doctorate from UMDNJ, now Rutgers School of Dental Medicine. She holds a second doctorate from Capital University of Integrative Medicine, Washington DC, and is a board-certified biological dentist and a past president of International Academy of Biological Dentistry and Medicine. She has lectured internationally on the Huggins-Grube Protocol. Besides holding several fellowships, she is the owner and CEO of DNA ConneXions, Biocomp Laboratories, Huggins Applied Healing, and Centers for Healing.
Anita Vazquez Tibau is a researcher at the Center for Environmental and Toxicological Research at the University of Puerto Rico. She has been working on a global ban on dental mercury for two decades, working with various non-governmental organizations. Since 2002, she has been an active participant in what is now called the Minamata Convention on Mercury treaty, where she wrote and delivered the closing statement on behalf of the International Academy of Oral Medicine and Toxicology. She has published articles in international journals, and several of her articles have been translated into multiple languages. She has lectured in the US and abroad. Most recently she was the recipient of the Humanitarian Award at the Doctors Who Rock Gala, 2019, Orlando, Florida.



