Joseph Shen, MD
Dear Readers,
We are thrilled to introduce a groundbreaking advancement in cardiac health assessment – the Multifunctional Cardiogram (MCG). In collaboration with esteemed experts, we present a remarkable non-invasive tool to aid heart disease diagnosis that promises to redefine how we diagnose and understand cardiovascular conditions.
The MCG represents a quantum leap forward in the early detection and timely monitoring of cardiac disease, offering a comprehensive and accurate assessment of heart function free from exposure to chemicals, radiation, and surgery. With its cutting edge capabilities, MCG provides information heretofore unavailable before now, even at the best teaching hospitals and elite practices at drastically more affordable costs and greatly decreased turnaround times, making diagnoses often otherwise missed by mainstream tools. With the MCG, the potential guarantee of early prevention is no longer a dream; it is a very real and present reality.
For those eager to witness the Multifunctional Cardiogram’s capabilities firsthand, we invite you to contact Amy Spahic at 904-624-8142 or Cami Braswell at 321-310-6644 CardioVision CARE or info@cardiovisioncare.com for available technology demonstration and testing services dates. Discover the future of preventive heart healthcare with this revolutionary technology.
Join us on this journey of innovation and advancement in the early detection and prevention of heart disease as we delve deeper into the Multifunctional Cardiogram’s transformative impact on your overall heart health in this story of an epic journey to Freedom of its physician inventor and creator.
Sincerely,
Joseph T. Shen, MD
Allow me to quote the first woman editor-in-chief of the New England Journal of Medicine:
“It is no longer possible to believe much of the published clinical research or rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor of the New England Journal of Medicine.” ― Marcia Angell28
And another, from Professor John Ioannidis, Professor of Medicine (Stanford Prevention Research), of Epidemiology and Population Health, and, courtesy of Statistics and Biomedical Data Science, in his Medscape interview, titled “Most research is flawed; let’s fix it”:
“Most of, nearly 90%, the four to five million peer review published papers in medicine, nutrition, economics, genetics, etc. are flawed. These papers have near-zero value in advancing science. Slightly more than ten percent are based on good evidence or mostly common sense.” ― John Ioannidis27
And, for good measure, a few more:
March 20, 2023: “Cardiologists received $1.1 Billion in industry payments in 6 years.” (The journal editor points out that leading to over-utilization of unproven drugs and devices is the bottom line.) -― Cardiovascular Business29
“In the U.S., the total expenditure in diabetic drugs, monitoring, and treatment reached $350 billion last year —10 percent of the overall healthcare expenditure. That’s the problem that Medicare for ALL, or any insurance paradigm, can’t fix. The system is broken not because of healthcare but because of health. And treating the symptoms isn’t enough, and we have to treat the root cause.” ― Robert H. Lustig, MD, MSL30
“They lie to us, we know they’re lying, they know we know they’re lying, but they keep lying to us, and we keep pretending to believe them.” ― Alexander Solzhenitsyn
A combination of systematic falsehoods and misleading information has caused catastrophic harm to the average American and inflicted unsustainable extreme costs to the American economy in trillions annually. As such, there is an existential need to pursue new innovative solutions that will prove highly accurate, trustworthy, and have the best outcomes at the lowest possible costs—cover all ethnicities and racial and economic backgrounds, seeking genuinely impactful information to benefit all.
A decentralized, carefully vetted, accurate, trusted, diversified, and widely distributed truth-seeking, telling-no-lies worldwide data network to incentivize honest and innovative work to benefit humanity is urgently needed. Such a data network system will deliver the means to counter the systemic issue of the medical industry’s peer-review published clinical validation trials designed to cherry-pick data to fit preordained narratives that benefit the device and pharmaceutical industry paymasters.
Sadly, such a system does not exist yet. Still, in the pursuit of honesty, transparency, and excellence of our creative digital heart disease diagnostic work beyond the conventional, when we created the MCG Technology, we consciously chose to avoid the business-as-usual methods and blaze our trail to deliver a tool that genuinely works to serve all of its patients as intended. We have accomplished that ourselves without quid pro quo and conducted ethically with 100% evidence-based, empirical, real-world data to train the deep machine learning A.I. system and perform our internal and third-party independent external clinical verifications and validations.
Coincidentally, on July 12th, 2023, Elon Musk and the company announced the startup XAI. The startup’s goal is to understand the true nature of the universe. Musk says that mainstream AI platforms must be built based on understanding the first principles and empirical evidence, supported with ethically responsible, objective, verifiable, and reproducible data. Rather, these commercially active platforms are vulnerable to propagating biased, false misinformation, or nefarious disinformation to mislead the masses and to the ultimate destruction of our civilization. The announcement reminds me of our journey started by my predecessors, professors in aerospace engineering, physiology, bioengineering, digital signal processing, and computer and data sciences, who decided to build a 100% empirical data and evidence-based AI system built upon the first mathematical and physiological principles, not on expert opinions without basis, decades ago!
As the leader of the second-generation developers of our platform, I wholeheartedly agree—an unimpeded-lying AI platform would have amplified and weaponized the lies from humans to such a degree that it would be horrifically destructive! Millions or even billions of people will be physically, mentally, legally, economically, morally, and spiritually injured, deeply betrayed like most US physicians feel right now! Increasingly, more physicians feel they have been indoctrinated to push drugs and devices for the big Pharma and device companies instead of caring and advocating for their patients to be their trusted “doctors,” violating their Hippocratic oath.
When I took over the lead in 1997 as the only physician team member in the group, AI was still in its winter; the earlier attempts to launch AI-based systems failed, precisely caused by the unsubstantiated claims of “efficacies” based on “expert opinions” and lack of foundational first principles and trusted vetted empirical data and independent third-party validation evidence to back up their claim. We decided to steer clear of the “business as usual” practices. We embarked on our long and arduous journey to adopt the new truth-telling “first principles and reality-based” paradigm to build a reality-based AI system that sets a new standard for building trustworthy and reliable tools to aid the diagnosis and deeper reality-based understanding of the true nature of human physiology.
This was, of course, neither an easy journey nor a short one, as the endeavor required an entire decade to hunt down like-minded, independent, equally honest, and ethically focused clinical investigators to validate the technology with the highest possible quality methods to instill trust in our separate and independent third-party validation process and restore people’s confidence and trust in the integrity of the scientific techniques that CAN be effectively and efficiently applied in medical research. We are working on a new, unprecedented digital, individually owned, and vetted/monetized decentralized and trusted high-quality data registry/network applying blockchain technologies to shatter this bottleneck.
In the words of Vijay Gupta, an engineer, teacher, and scientist:
“Empirical evidence in medicine (and nutrition science) comes from various sources. Each source has its pros and cons.….Only large companies can afford to conduct large RCTs. But that evidence is tainted because the motivation behind that research is profit, not science. Drug companies are not required to report raw data or failed trials. This amounts to cherry-picking data that essentially invalidates their evidence. Moreover, the subjects used in RCTs may not represent you biologically or otherwise. They may have different ages, races, gender, or health status. Finally, the two sigma standard is pathetic.”[31]
But how do we avoid the pitfalls that large-sized (a few thousand cherry-picked patients) RTCs, able to pay prices between $5,000 to $20,000 per patient for their efforts, fall into?
Firstly, all evidence we collected had to be performed through controlled, blinded observational trials, using diversified, distributed, and unbiased data sources from Asia, Europe, and North America to deliver our goal of understanding the performance of the MCG Technology Platform in real-world circumstances, comparing it against platinum standard diagnostic modalities.
We hired an experienced independent data monitor to ensure everyone participating was honest and the datasets were transparent and readily available for independent verification by the doubters or collaborating investigators. All this guarantees that the technology WILL work in the real world on actual patients.
We also welcome people to replicate these results and find new targets to pursue, such as women with the infamous “Syndrome X” or potentially microvascular disease due to metabolic heart disease and detection and monitoring to the earlier stages of heart failure of any cause, room for potential disease reversal, etc.
As Professor Vijay Gupta pointed out: “The Evidence is much cheaper and more plentiful. It is more unbiased. Noise or errors are random and cancel out over time (as opposed to a cherry-picking bias which is one-sided). Dr. Nigam Shah of Stanford has made some excellent discoveries by analyzing an extensive database of personal health records of past Stanford patients.”31
As such, layer by layer to build our unique digital neural network, our approach has given us profoundly meaningful 100% empirical evidence-based training and verification/validation of deep supervised machine learning datasets, with a level and degree of quality that allows meta-analysis17 of the replicable and reproducible data to prove our credibility to the FDAs of the USA, Japan, South Korea, Malaysia and beyond, along with a unique CPT Code description, Medicare Coverage with a local coverage determination policy, adopting our MCG Technology Platform as a replacement for all the legacy analog cardiology imaging tests in their arsenal.
We have since gone to market and received worldwide adoption, serving more than 3 million individuals, saving tens of thousands of lives, and extending the lifetimes of millions. The adoption rates, meanwhile, only continue to accelerate, and despite continuous attempts by the establishment of legacy medicine to impede our progress, we have continued to thrive.
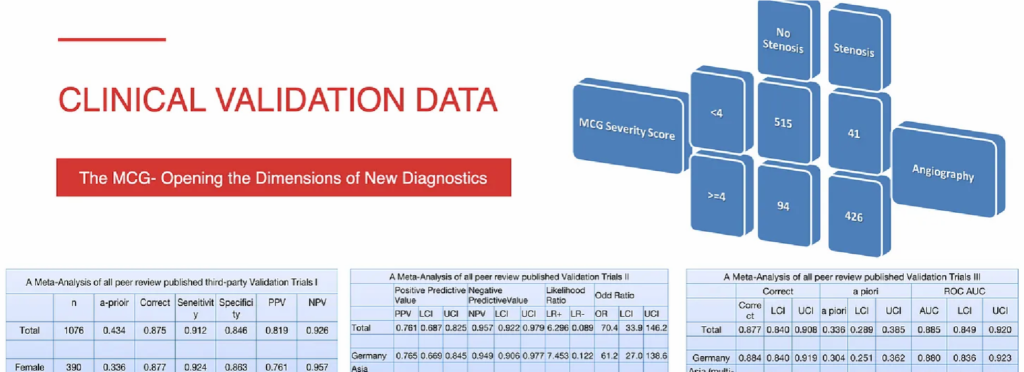
The validation of MCG in the detection of myocardial ischemia caused by obstructive coronary artery disease (CAD) has been demonstrated in multiple independently conducted clinical trials collecting data from diverse populations in eight countries13-25 with high sensitivity (89-100%), specificity (83-94%), and negative predictive values over 95%, with only ONE outlier, which was later retracted.23-25
The accuracy above can be improved when the results of MCG are correlated with serum biomarkers such as abnormal fasting glucose, hemoglobin A1c, high levels of triglyceride vs. low HDL, hyperinsulinemia/Insulin-resistance, fatty liver (ALT >25), and another newer heart failure marker, hBNP.
When independent investigators examined the data sets in further detail, they also discovered that the specificity improved dramatically (89% to 95%) if tighter criteria (from an FFR cutoff value of less than 0.8 to less than 0.75) were applied. The details of each study will not be discussed here, but a few should be highlighted.
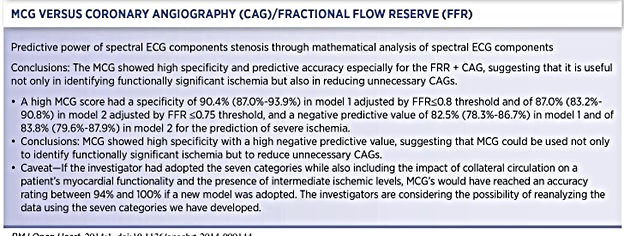
The landmark clinical validation of MCG was a study by Amano, Shinoda, Kunimura et al20 ( see Table 2). The study was done in Japan but published in the Open Heart Journal of the British Medical Journal. They combined angiographic and functional flow reserve (FFR) data. They demonstrated that MCG has high specificity and negative predictive value. They concluded that it could be used to identify functionally significant ischemia and reduce unnecessary angiograms.
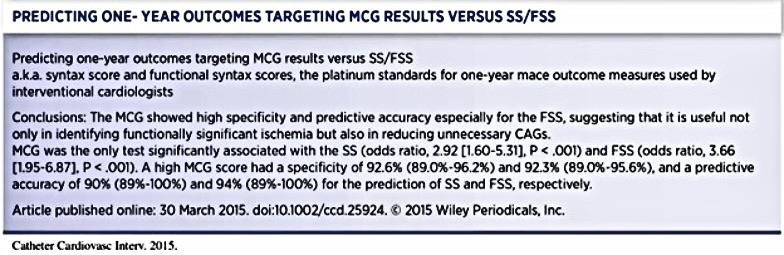
Another Japanese study by Takeshita and Shinoda compared classic syntax scores (SS) with functional syntax scores (FSS) 21 (see Table 3). The SS is derived purely from anatomical analysis of angiographic data, and the FSS is derived from adding fractional flow reserve (FFR) information to the study. FFR identifies significant falls in pressure recordings across both apparent obstructive and not-so-obvious nonobstructive lesions. They concluded that MCG showed high specificity and predictive accuracy, especially for FSS, again supporting MCG’s usefulness in identifying functionally significant ischemia and its potential role in reducing unnecessary catheterizations.
Another important event was a poster presentation of an outcome predictive study at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) meeting in San Francisco.22 TCT is a prestigious American society for interventional cardiologists. The poster by Tetsuya Amano, Norihiro Shinoda, et al.22 showed that MCG correctly identified all six cases of (6) restenosis and two (2) new blockages with a sensitivity of 94.3% and a specificity of 97.3% among 720 epicardial coronary artery segments of 45 consecutive patients who were followed one-year post placement of coronary stents. MCG delivered a 0.94 (0.89 to 1.0) in the area under the receiving operating curve (ROC) analysis to predict adverse events.
Finally, a word on the only outlier among these trials: Kawaji et al. published a study allegedly showing a poor correlation between angiographic with FFR and MCG.23 However, there were several problems with the significant deviation from the original protocol.24,25 They also did not consider collateralization as a cause of false negative results. Also, the decision not to do FFRs on many people with diabetes with non-obstructive diseases inserted a significant degree of bias. We, unfortunately, have some cause to believe this was an attempt to discredit our work. However, we thankfully designed and implemented a twin site using the identical protocol to ensure the replicability and reproducibility of our output. This twin study was accepted for peer-reviewed publication, and British Medical Journal accepted the manuscript and published it in its Open Heart Journal.20 This unforgettable honorable event highlighted our decade-long efforts in this in-depth investigative clinical validation series.
The authors of the outlier piece, after learning that some Americans arrived at Kyoto University in what was potentially an attempt to interfere with the trial implementation process, reanalyzed their data and issued a retraction, determining that cases they initially regarded as false positives were true-positive cases in detecting heart failure, myocardial damage post total coronary occlusion, etc. This gave our validation efforts an unprecedented, 100% positive, unblemished record, a historical outcome in Western medical device validation and peer-reviewed literature.13-25,26
This excellent outcome allowed us to close the chapter on the most robust and outstanding independent third-party verification and validation evidence gathering efforts, with the evidence we presented being the strongest in the history of CPT applications among ALL the stress imaging modalities approved for CAT I CPT codes to date.
It is evident that MCG’s capabilities for detecting early, intermediate, and late-stage metabolic-based myocardial ischemia and natural recovery stages go far beyond conventional diagnostic stratagem and are of particular value in women or individuals with microvascular or non-obstructive diseases, such as diabetes mellitus. MCG has also been demonstrated to have a direct and close correlation with the physiologic FFR measurement. MCG provides a uniquely positioned high-quality diagnostic tool for clinicians who are making critical diagnostic and clinical management decisions in a timely, affordable, and dependable manner at the patient’s bedside in real-time.
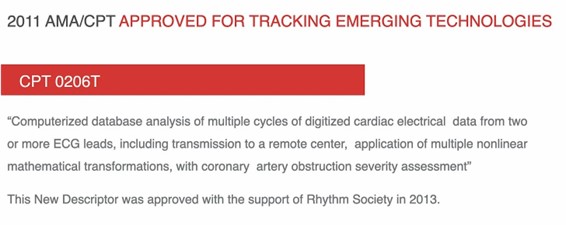
Following the publications of the clinical validation trials, The American Rhythm Society supported our application for a unique CPT code because of the overwhelming superior evidence we presented. However, the cardiologists sitting in the leadership positions of the CPT editorial panel did not grant MCG the well-deserved CAT I Code; instead, they placed politics ahead of better patient care and manipulated the system to downgrade our CPT Code application to a CAT III Code, 0206T (See Figure 1). The capitalized letter “T” is like a dog whistle, which means “Temporarily designated” to buy time until they eventually push us out of the market.
This was when I learned that the entire CPT/Reimbursement System is rigged and not based on scientific merit but on whom you know and political connections in a monopoly game. Regardless, we applied for reimbursement via local and regional Medicare carriers. Surprisingly, the independent-minded Medicare medical directors reviewed our published evidence and desired to cover our technology regardless of the determination from above. Their decision to issue a draft of the “Local Coverage Determination” policy (see Figure 2) caused a surge in adoption of the technology in the covered states, New Jersey, Delaware, Maryland, and Washington DC; however, it also led to a firestorm eruption among the card-carrying members of the American College of Cardiology. Please note the timing of these events; they are not accidental or coincidental, frankly.

The racket of lobbyists, politicians, ACC lawyers, and AIM cardiologists embedded in the insurance companies, especially the BCBSs, and characters throughout the revolving doors of the US regulatory agencies in Washington DC, or state capitols, came out swinging in attempts to sink our ship. And for ten (10) years, our little family-owned business prospects turned truly dark and frighteningly hopeless, and our survival was in terrible jeopardy.
However, refusing to retreat, my family members, the development team, and I forged forward and found ways to thrive. We are incredibly grateful that hundreds of early adopters and millions of patients worldwide supported our work through those times! Every day, new customers join our extended family; these patient-centric care physicians, physician assistants, and nurse practitioners have enabled our technology to shine! Additionally, our efforts to extend the understanding and increase the applications of the technology have NEVER ceased–such as investigations on how MCG can help patients with chest pain presenting at emergency departments with low risks of cardiovascular disease either avoid unnecessary hospital stays or being sent home prematurely due to false negative testing results from the ERs.
Additionally, independent investigators have demonstrated great promise in trials that MCG can help predict recurrent atrial fibrillation post pulmonary vein isolation or the PVI/Ablation procedures. A five million people data registry has been designed and planned to develop a 21st Century “Heart Study” to advance the legacy of the “Framingham Heart Study” based on early detection, lifestyle optimization, and 100% empirical evidence-based decision-making for the best short, intermediate, and long-term outcomes at the lowest possible cost. We are ushering in the era of safe and effective bedrock 100% evidence-based patient-centric lifestyle optimization medicine to deliver the best possible healthcare for future generations.
MCG Technology will serve as the tip of the spear of patient-centric digital medicine to allow objective detection, quantification, and monitoring of every patient using it at an affordable cost. Our work will contribute to a new world of safer, trustworthy, and effective cardiovascular disease early detection, and primary prevention to prolong healthier, productive, satisfying, and active lives for tens of billions of earth’s inhabitants and beyond.
Next, to satisfy your more serious due diligence need on behalf of patients, and again in the interest of public health, I will provide all the published evidence up to date for your convenience below:
A meta-analysis of all published trials performed and analyzed in the US, published in the International Journal of Medical Sciences 2009; 6(4) pp. 143144. Without any traditionally accepted quid pro quo, this summary includes statistical information from clinical trials between 2000 and 2004, representing a sample of more than 1,000 patients in three major geographic regions (North America, Europe, and Asia). All studies in this group were performed per Premier Heart’s Westchester Study Protocol, utilizing standard double-masked testing and independent verification of results. Overall sensitivity across these studies was 91 percent (specificity 83.8 percent, positive predictive value 77.6 percent, negative predictive value 92.4 percent).17
The first ever published clinical validation trial conducted at Westchester Medical Center, New York, USA—again, without quid pro quo, or conflict of interest. The results were published in Heart Disease 2002; 4: pp. 2-12. This study compared the results from MCG with the results of coronary angiograms in a prospective, double-masked fashion random sample of 136 patients. This study showed a sensitivity of 93.3% (specificity 83%, positive predictive value 91.2%, negative predictive value 86.7%).13
Siegburg Heart Center (Siegburg, Germany): Two studies were performed at the Siegburg Heart Center in Siegburg, Germany, evaluating MCG in patient populations with and without a revascularization history.14,15 The first study evaluated the use of MCG in 423 patients with no prior history of coronary revascularization; results were published in International Journal of Medical Sciences 2007 4(5): pp. 249-263. In this study, our technology showed a sensitivity of 89.1 percent (specificity 81.1 percent, positive predictive value 79 percent, negative predictive value 90 percent).
The second study evaluated the ability of our technology to deal with the unique challenges of patients with a prior history of coronary revascularization, testing a sample of 172 patients; results were published in the International Journal of Medical Sciences 2008 5(2): pp. 50-61. In this study, our technology showed a sensitivity of 90.9 percent (specificity 88 percent, positive predictive value 62.7 percent, negative predictive value 97.8 percent).
Asian Multi-Center Study – Four Sites. Results were published in Congestive Heart Failure 2008 14: pp. 251-260. This study was conducted across four centers in Asia with a sample of 189 patients (including patients with and without a history of prior revascularization). This study yielded a sensitivity of 94.8 percent (specificity 86.6 percent, positive predictive value 78.4 percent, negative predictive value 97.1 percent).16 The results were reproducible and method replicable throughout all the centers.
A Single-Center Study of 116 Consecutive Patients: results were published in the International Journal of Medical Sciences 2011; 8(8):717-724. Symptomatic patients with known or suspected coronary disease and valvular heart disease (VHD) agreed to undergo an ethical and responsible triple-blinded, prospective, controlled study with MCG Testing, Stress SPECT MPI with sestamibi, resting TTE, and a coronary angiogram (C.A.). This study yielded a sensitivity of 91 percent and specificity of 85 percent by applying MCG Technology, while the MPI performed very poorly, generating specifies as low as only 8%!18 The overall accuracy of MPI was well BELOW 50%, validating the reality presented by Patel and Dharmarajan.18
A Meta-Analysis published in Treatment Strategies – Cardiology Volume 3, Issue 1, pp.829. Data from three published trials of the use of MCG in identifying relevant coronary stenosis was used in this meta-analysis.19 Three hundred ninety female patients scheduled for coronary angiography were included in the meta-analysis. Coronary angiographic data were reviewed by two independent interventional cardiologists, blinded from the MCG results, and the MCG Technology was blinded to the angiographic results; therefore, vice versa. This study yielded a sensitivity of 92.4 percent and a specificity of 85.3 percent-consistent with earlier reports of the technology.24
Another pivotal trial, “Noninvasive Assessment of Functionally Significant Coronary Stenoses Through Mathematical Analysis of Spectral ECG Components,” was conducted and published to validate and demonstrate the MCG’s capability to generate even better specificity, thus, better accuracy.20 This one was performed parallel to the ONLY outlier of the body of validation work on MCG, the Kimura study (see below), which was cherry-picked as the only negative reasoning for coverage denials! Results were published in Open Heart 2014;1: doi:10.1136/openheart -2014-000144.
A consecutive 112 participants suspected to have CAD who were scheduled for elective coronary angiography (CAG) from October 2012 to December 2013 were examined.20 Their predictive values of relevant ischemia were compared by MCG, standard ECG, and Framingham Risk Score (FRS). In conclusion, the MCG showed high specificity and high NPV regardless of gender in relatively high-risk patients, suggesting that the MCG could identify relevant severe ischemia. In addition, the potential use of MCG in evaluating ischemic CAD appeared more feasible than standard ECG and FRS. This was the parallel replicating twin study conducted alongside the outlier by Kimura et al23 : “Noninvasive Detection of Functional Myocardial Ischemia: Multifunction Cardiogram Evaluation in Diagnosis of Functional Coronary Ischemia Study.”
MED-FIT (or as I called it a “MED-HIT”) was the only outlier of the entire body of MCG technology validation evidence. A rebuttal Letter to the Editor appeared in Noninvasive Electrocardiology Journal (2015): “It All Depends on Your References: Electrophysiology Compared to Angiography” by Michael Imhoff, MD, PhD, from the Department for Medical Informatics, Biometrics and Epidemiology, Ruhr-University, Bochum, Germany, and QTEC-group, Lübeck, Germany, and Norbert Rainford, MD. Drs. Imhoff and Rainford pointed out the methodological deviations of the Kimura team from the agreed-upon protocol and their selective cherry-picking of much older patients who survived critical coronary ischemia due to occlusive CAD and developed collateral circulations skewed the results they reported.24
To put the controversy to bed, the same dataset was analyzed the second time by the same people of the Kimura paper who published a ‘retraction sort of paper and concluded that MCG is good for “screening for early detection of CAD,”25 but in Japanese: 冠動脈硬化スクリーニングにおけるMultifunction CardioGram (MCG側) の有用性の検討 Validation of Multifunction CardioGram MCGTM in the screening of Coronary Atherosclerosis 川治 徹真 塩見 紘樹 西川 隆介 矢野真理子 樋上 裕起 田崎 淳一 今井 逸雄 斎藤 成達 牧山 武 静田 聡 尾野 亘 木村 剛.
An independent bio-statistician reanalyzed the same dataset based on the correct original trial design and the analysis method, applying the new high categories criteria for disease severities developed by Joseph T. Shen, MD et al.. The investigators independently concluded the following: the more strict the cutoff of the FFR, the higher the specificity of the automated and unbiased objective MCG test reported results (see Figure 3).
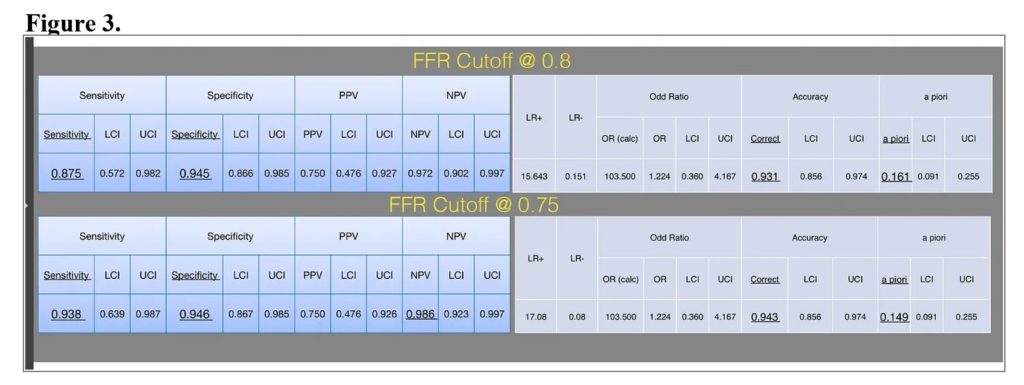
Regardless of the relentless resistance, life continues, and the MCG development team and clinical validation investigators make progress.
To further demonstrate the MCG Technology’s prowess in its power to aid better diagnosis, another excellent trial was designed: “Noninvasive Mathematical Analysis of Spectral Electrocardiographic Components for Coronary Intermediate Lesions to Obstructive Stenosis Severity – Relationship with Classic and Functional Syntax Score.”21 The method was unprecedented: comparing the results of MCG directly to Classical and Functional Syntax Scores! This type of comparison was unheard of in the history of decal device validation and remains so. Results were published in Catheterization Cardiovascular Interventions, Volume 86, Issue 1, July 2015, Pages 21–29.
This study aimed to evaluate the accuracy of the MCG concerning the SS and FSS in a relatively high-risk population who were scheduled for elective CAG, taking into account standard ECG and Framingham risk scores (FRSs). The significant findings in this study are that the MCG score was significantly associated with the SS and the FSS in a relatively high-risk population with or without known CAD. Furthermore, the high MCG scores showed relatively high predictive accuracy for high FSS. Without any quid pro quo, the investigator concluded that “these findings could have significant clinical implications on the improvement of noninvasive diagnosis tests in terms of the diagnosis of relevant ischemic heart disease.”21
There was NEVER any mention of MCG being questioned for the poor quality and quantity of validation evidence accumulated and published in the medical literature. So why did the AIM cardiologist make irresponsible statements questioning the quality of the work? The motivation must be examined and investigated.
Japanese Restenosis Outcomes Trial (2015 – 2020): A multi-center clinical trial, including Aichi Medical University, Chubu Rosai Hospital, and the Nagoya University Graduate School of Medicine, studied the impact of noninvasive mathematical analysis of spectral electrocardiographic components on the prediction of recurrent cardiac ischemic events after coronary intervention. Forty-five consecutive patients who underwent coronary intervention were enrolled and analyzed using the Multifunction Cardiogram to study its effectiveness as a predictor of recurrent cardiac ischemic events. The initial conclusions indicated that “the MCG may be a useful predictor of recurrent ischemic events after coronary intervention, especially in patients who cannot exercise and have low kidney function.”21
The EMAC Study from 2016 to 2020 was the first report presented at ACEP Virtual Latest Breaking Trial Presentation in 2020 by Linda Papa, MD et al., Orlando Healthcare System: EMAC Study First Dataset Presentation
Study Objectives: Emergency physicians (EPs) could benefit from a noninvasive, costeffective, and accurate tool to determine which patients with suspected acute coronary syndrome (ACS) have significant coronary obstruction. A novel computerized, multiphase, resting electrocardiogram analysis device, the Multifunction Cardiogram (MCG), combines the traditional 12-lead ECG with mathematical modeling and functional measurements of the heart’s electrical activity. Independent studies in patients with high-risk coronary artery disease have shown the MCG to have a high correlation with coronary stenosis confirmed by angiography. This case series aimed to describe the application of the MCG to low-risk chest pain patients with suspected ACS presenting to the E.D. and compare results to angiography.
Methods: This prospective study enrolled a convenience sample of adult patients presenting to a tertiary care academic teaching center with chest pain in whom the E.P. suspected low-risk ACS. Patients with ECGs showing active ischemia (including STEMI and NSTEMI) and those unable to complete follow-up were excluded. After evaluation by the E.P. and obtaining informed consent, an MCG was performed. To get the MCG reading, data from two traditional ECG leads, lead II and V5, were collected for 82 seconds, and 3-5 tests were performed on each patient. MCG results were electronically transmitted to a central computer, where the data was mathematically transformed and analyzed to identify distinct functional indices. A risk score ranging from 0 (minimal risk) to 20 (very high risk) was provided. The E.P. was blinded to the MCG results, which did not change medical management. The outcome was based on the coronary angiogram results, either Coronary Computed Tomography Angiography (CCTA) or conventional angiography. Angiogram results were classified as no coronary artery disease (CAD), mild CAD, moderate CAD, and severe CAD based on the degree of stenosis subjectively visualized by the cardiologist.
Results: There were 511 patients enrolled with a mean age was 52 (SD23), and 51% were female. 1% were Asian, 18% Hispanic, 33% African American, and 47% White. Of these, 47 patients (9%) had an angiogram performed (63% CCTA): 23 (49%) had no CAD, (12) 26% had mild CAD, 7 (15%) had moderate CAD, and 5 (11%) had severe CAD. The mean MCG score for patients with no CAD was 2.3 (95%CI 1.0-3.1), mild 2.6 (95%CI 0.8-4.3), moderate 3.4 (95%CI 1.2-5.6), and severe 4.8 (95%CI 1.4-8.2). Angiogram results were dichotomized into powerful and non-severe. Mean MCG scores in patients with severe CAD were 4.8 (95%CI 1.4-8.2) compared to non-severe CAD 2.4 (95%CI 1.6-3.2)(p=0.046). The mean of the highest MCG score was 6.8 (95%CI 4.39.3) for severe CAD and 3.1 (95%CI 2.2-4.0) for non-severe CAD (p=0.006). The area under the ROC curve (AUC) for predicting severe CAD was 0.76 (95%CI 0.57-0.95) using the average MCG score and 0.85 (95%CI 0.70-0.99) using the highest MCG score. The sensitivity of the highest MCG score for predicting severe disease using an index score of 4 or greater was 100% (95%CI 46-100%), specificity was 56% (95%CI 40-71%), negative predictive value 100% (95%CI 82-100) and likelihood ratio 2.3 (95%CI 1.6-3.2).
Conclusions: MCG scores increased with the severity of coronary obstruction. This study introduces the MCG as a potential tool for assessing low-risk chest pain patients with suspected ACS in the E.D. A large prospective multi-center study is ongoing.
AICHI Atrial Fibrillation Study (2016 to 2020, and beyond) – A clinical trial led by the Aichi Medical University (Nagakute, Japan) is currently underway to examine the ability of the Multifunction Cardiogram to predict recurrence of atrial fibrillation after pulmonary vein isolation procedures. A total of 39 consecutive patients receiving pulmonary vein isolation were enrolled. Recurrence of A-fib (N=6) was defined as within three months after pulmonary vein isolation. Preliminary results show that the frequency of MCG reporting “arrhythmic tendency” after pulmonary vein isolation was significantly higher in recurrent A-fib. The MCG appears to be a valuable predictor of A-fib after pulmonary vein isolation.
The follow-up outcomes study of the original Aichi Atrial Fibrillation Study (2016 to present) is as the following:
Objective: To evaluate the potential ability of the Multifunctional Cardiogram (MCG), an A.I./deep machine learning digital signal processing, empirical data-mining method based on the multiple mathematical functions applying the principals of Lagrangian mechanics, to classify groups with recurrence of atrial fibrillation (A.F.) after pulmonary vein isolation (PVI) procedures.
Methods: This prospective single-center observational study included data from the cardiac catheter database of Aichi Medical University collected between xxx and xxx. Among consecutive xxx patients with A.F., the data for 83 patients who underwent both catheter ablation (C.A.) with PVI and MCG were analyzed. A.F. recurrences were defined as “late” and “very late,” occurring 4-12 months after PVI. Patients were divided into the recurrence group (R) and the non-recurrence group (N.R.). The MCG analysis consisted of a series of Regression vs. Classification: The preliminary data analysis consisted of linear and logistic regressions. These included Linear Regression, Polynomial Regression, Elasticnet, and LASSO. The regressions were performed on a subset of the per-sample MCG features known as the independent feature set, using number-of-recurrences as the outcome variable. The simple Linear Regression did not prove predictive (<= 50% match), while the more sophisticated regressions were slightly better (50-60%). The experiment was modified to use Logistic Regression, with the same feature set, using the boolean recurrence variable (0 if there was no recurrence, one otherwise) as the outcome variable. This fared better (60-65%), which led to the redefinition of the problem as one of classification (R vs. N.R.) rather than prediction. The revised goal suggested using a classifier, a Decision Tree, and a confusion matrix classifier was used to good effect (70-90%), albeit subject to over-fitting. The final and best-performing analysis method was the best model applied to the dataset per-sample confusion matrix result reporter. This performed well, though susceptible to false negatives (R classed as N.R.): providing an accuracy of 94%, T.P. 76 F.P. 10 TN 258 FN 12
Results: Of 83 patients, a recurrence of A.F. occurred in 24 patients (29%). The best performer was RandomForestClassifier delivering an accuracy of 94%, T.P. 76 F.P. 10 TN 258 FN 12
Analysis: The approach would likely produce a more accurate model using a larger, more balanced, and granular dataset, such as early (3 months) vs. late (12 months) recurrence cases.
Conclusions: MCG can be potentially useful as an objective tool to create an algorithm to classify a recurrence of A.F. after PVI. Large-scale longer-term outcomes data validation trials are being planned.
Our missionary work never ceases.
What is a more straightforward explanation of what MCG Technology does? There is this: We build the mathematical “brain” to first learn what would cause the physiological supply and demand imbalance expressed by the mitochondrial biological neural networks, such as ischemia, myocardiopathy, various structural anomalies, myocardial inflammation, myocarditis, pulmonary diseases, metabolic dysfunctions, neurohormonal disorders, environmental conditions, toxic substances, etc.
We carefully adopted diligently vetted real-world patient practical training datasets to develop machine learning algorithms to allow the “brain” to accurately identify these discovered functional patterns in new patient data sources to optimize, perfect, verify, and validate the accuracy of our algorithms independently.
The hard work we have put into developing the technology is to ensure that this “A.I. Physician Diagnosis Assistant” shall never act on its own to usurp the decision of its human masters and go rogue.
Our mathematical “brain” is 100% empirical and evidence-based by design and development processes, with uncompromising work integrity and dedication to our patient-centered goals.
MCG Technology delivers the critical physiological functional information missing in the current legacy cardiovascular disease detection arsenal for safe and effective early detection and impactful clinical decision-making to timely and cost-efficiently diagnose, prevent, treat, and reverse cardiovascular diseases whenever possible.
Finally, this is my Vision for a Better and Brighter Future: We learned from our experience developing the MCG technology; obtaining high-quality and trusted real-world data has been the bottleneck. The process is time-consuming, costly, and highly labor-intensive. The ultimate system is decentralized, which allows individualized digital tokenized data ownership, monetized and tokenized, as the data collectors, verifiers, and handlers enrich the raw data, including physician input.
Everyone who works on building the perfect data profile will receive compensation each time a customer uses the enhanced datasets for any reason, including clinical trials. Our technology will spearhead this distributed data network for reporting honest, trustworthy, and faithful evidence.
This setup will allow nearly real-time reporting, analysis, and deep digital learning, significantly speeding up the discovery by adopting edge computing to our digital signal processing mathematics, empirically data-driven diagnostic software algorithms, and deep machine learning. We will add additional neural network deep learning techniques via programmable GPUs, such as recurrent and reinforcement neural network layers superimposed on the data network, to deliver a “keeping-everyone honest” and trustworthy decision-making information system that produces the lowest possible cost for the highest quality and the best outcomes for everyone.
People can make structural improvements confidently on the objective and unadulterated independent protocol replication, verification, and validation of the safety and effectiveness of any procedure, drug, or device, old or new. We can retake our profession back! We can reestablish our freedoms and regain trust by minimizing/eliminating cherry-picking of data to fit preordained paid narratives by the paymasters of the legacy medical-industrial complex to corrupt the system.
Caregivers will achieve professional satisfaction and financial independence without too much interference from the middleman. A network everyone has access to and weighs in without the fear of censorship and discrimination.
Everyone can access this network/data market without fearing censorship, disruption, marginalization, ridicule, and discrimination. Instead, all voices will receive the due attention and evaluation based on their merits and be treated with mutual respect and common interest—the goal is to deliver the best possible care for everyone, with the best possible outcome and at the lowest potential costs, including humans, plants, and animals, every life, on earth and beyond.
Our hard work has created a solid foundation model for the discipline of computational systems electrophysiology supported by the world’s best computing hardware platforms, data scientists, and software developers. More applications can be developed from this foundation, for example, the computational systems cerebral electrophysiology platform, pediatric applications, maternal-fetal-medicine applications, space medicine, lifestyle medicine, new anesthesia system, new drug discovery, safety and effectiveness validation for ALL nutrients, drugs, environmental toxins, all treatment options, conservative or interventional, etc. The future is extraordinarily bright and hopeful! We believe in our work and inspire the generations to join our journey, and we look forward to the exciting journey ahead way into the future!
This decentralized, ethical, accurate, trusted, safe, and effective data worldwide marketplace will be a better place for ALL! Welcome to the safe and effective lower-cost digital health of the 21st century!
Finally, on the eve of Nobel Prize Winner W. Einthoven’s first EKG machine in 1901, 122 years later, we celebrate the birth of its 21st Century replacement, “Multifunction Cardiography Technology,” or the MCG A.I. Technology Platform. We welcome everyone to join the celebration, especially those who have read ECGs for a lifetime, the legacy electrocardiologists!
The National Academy of Medicine of the National Academy of Sciences advises major medical stakeholders in the United States on healthcare policy. Twenty-two years ago, they recognized that we still needed to change our approach to chronic diseases to manage them more effectively. They wrote a guidebook, “Crossing the Quality Chasm.”32 The name was no accident. They called out the failure explicitly. “The American health care delivery system needs fundamental change…Quality problems are everywhere, affecting many patients. Between our health care and the care we could have lies not just a gap but a chasm.”
Time to overcome the divide and join us, the pioneers of a digital revolution in the new world of the highest-possible-quality, the best-possible-outcomes, the lowest-possible cost affordable, and 100% empirical evidence-based practice of medicine for everyone to restore patient trust and faith it in our beloved profession!
Editor’s note: This article, Part 2 of three parts, is an edited and updated version of Chapter 12, “Multifunction Cardiogram, a.k.a. MCG” By Joseph Shen, MD (Raffi Bianchi Shen, editor) in Mark Houston’s book Personalized and Precision Integrative Cardiovascular Medicine (1st Edition). Part 1 was published in the June 3, 2023, issue of Townsend e-Letter.
References
- Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25.
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J 2014; 35:2950.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics2018 Update: A Report from the American Heart Association. Circulation 2018; 137:e67.
- Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999; 353:89.
- Patel MR et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362: 886-95
- Falk E, Shah, Fuster V. Circulation 1995,92: 657-671
- Sachdeva et al., Am. Heart 5 2009; 157(1) 111-117,e2
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J.Am. Coll Cardiol 2005:46(7): 1225-1228
- Sedlis SP, Hartigan PM, Teo KK. Effect of PCI in long-term survival in patients with stable ischemic heart disease. N Engl. J Med 2015;373:1937
- Tonino et al. New Engl J Med 2009;360:213-24
- Sarno G, Decraemer I, Vanhoenacker PK, et al. On the appropriateness of noninvasive multi-detector computed tomography coronary angiography to trigger coronary revascularization. J Am Coll Cardiol. Intervent. 2009; 2:550-557.Sachdeva et al., Am. Heart 5 2009; 157(1) 111-117,e2
- Ludwig von Bertalanffy’s General System Theory: Foundations, Development, Applications (Revised Edition) ISBN-10: 0807604534 | ISBN-13: 978-0807604533 | Publication Date: March 17, 1969, | Edition
- Weiss WB et al. Computer-enhanced frequency-domain and 12-lead electrocardiography accurately detect abnormalities consistent with obstructive and non-obstructive coronary artery disease. Heart Dis 2002;4:2-12
- Grube E, et al. Computerized two-lead resting electro-myocardium analysis to detect coronary artery stenosis. Int J Med Sci 2007; 4:249-263.
- Grube E et al. Computerized two-lead resting electro-myocardium analysis for detecting coronary artery stenosis after coronary revascularization Int. J. Med. Sci. 2008, 5 (2):50-61
- Hosokawa J et al. Computerized 2-lead resting ECG analysis for detecting relevant coronary artery stenosis compared with angiographic findings. Congestive Heart Failure 2008 14: 251-260
- Strobeck JE et al. Comparison of a two-lead, computerized, resting ECG signal analysis device, the MultiFunction-CardioGrams or MCG (a.k.a. 3DMP), to quantitative coronary angiography for the detection of relevant coronary Artery stenosis (>70%) – A Meta-analysis of all published trials performed and analyzed in the U.S. International Journal of Medical Sciences 2009; 6:143-155
- Strobeck JE et al. A Paired-Comparison of the MultiFunction Cardiogram (MCG) and Sestamibi SPECT Myocardial Perfusion Imaging (MPI) to Quantitative Coronary Angiography for the Detection of Relevant Coronary Artery Obstruction (≥70%) – A Single-Center Study of 116 Consecutive Patients Referred for Coronary Angiography International Journal of Medical Sciences, 2011; 8(8): 717-724
- John E. Strobeck, Norbert Rainford, Bonnie Arkus, and michael Imhoff. Treatment Strategies (2010) Comparing Multifunction-Cardiogram and Coronary Angiography for Detection of Hemodynamically Relevant Coronary Artery Stenosis (>70%) in Women.
- Amano T, Kunimura A, et al. Noninvasive assessment of functionally significant coronary stenoses through mathematical analysis of spectral ECG components. BMJ/Open Heart 2014;1:e000144. doi:10.1136/openhrt-2014-000144
- Shinoda et al. Noninvasive Mathematical Analysis of Spectral Electrocardiographic Components for Coronary Lesions of Intermediate to Obstructive Stenosis Severity–Relationship with Classic and Functional SYNTAX Score. Catheterization and Cardiovascular Interventions DOI 10.1002/ ccd. Published on behalf of The Society for Cardiovascular Angiography and Interventions (SCAI)
- Tetsuya Amano, Norihiro Shinoda, et al. Impact of noninvasive mathematical analysis of spectral electrocardiographic components on predicting recurrent cardiac ischemic events after coronary intervention, An Abstract submitted for 2015 PCI TCT San Francisco.
- Tetsuma Kawaji, MD; Hiroki Shiomi, MD; Takeshi Morimoto, MD, Ph.D.; Ryusuke Nishikawa, MD; Mariko Yano, MD; Hirooki Higami, MD; Junichi Tazaki, MD; Masao Imai, MD; Naritatsu Saito, MD, Takeru Makiyama, MD, Satoshi Shizuta, MD; Koh Ono, MD; and Takeshi Kimura, MD. Noninvasive Electrocardiology Journal (2015) Noninvasive Detection of Functional Myocardial Ischemia: Multifunction Cardiogram Evaluation in Diagnosis of Functional Coronary Ischemia Study (The “MED-HIT” Piece?)
- Michael Imhoff, MD, PhD; Norbert Rainford, MD. Noninvasive Electrocardiology Journal (2015). A Rebuttal Letter to the Editor: It All Depends on Your References: Electrophysiology Compared to Angiography.
- A Japanese Style Retraction: (In Japanese only, translated) Validation of Multifunction CardioGram(MCG) in the screening of Coronary Atherosclerosis. Tetsuma Kawaji, MD; Hiroki Shiomi, MD; Takeshi Morimoto, MD, Ph.D.; Ryusuke Nishikawa, MD; Mariko Yano, MD; Hirooki Higami, MD; Junichi Tazaki, MD; Masao Imai, MD; Naritatsu Saito, MD, Takeru Makiyama, MD, Satoshi Shizuta, MD; Koh Ono, MD; and Takeshi Kimura, MD.
- Access to ALL of the peer review published records in this historical journey: https://www.dropbox.com/sh/a9pg6te0ifn9o16/AAAVM-ZqeJbgs1E4kZJjgPyOa?dl=0
- John Ioannidis, “Most Research is Flawed; let’s fix it.” https://www.medscape.com/viewarticle/898405
- Marcia Angeles, the first woman editor-in-chief of the famous New England Journal of Medicine: https://www.bmj.com/content/346/bmj.f3830/rr/652673
- https://cardiovascularbusiness.com/topics/healthcare-management/healthcare-economics/cardiologists-received-11b-industry-payments-6?utm_source=newsletter&utm_medium=cvb_weekend
- Robert H. Lustig. Metabolical -The truth about processed food and how it poisons people and the planet. 2021 https://robertlustig.com/metabolical/
- https://www.quora.com/profile/Vijay-Gupta/posts
- https://substack.com/redirect/99823c5e-b3ce-4fce-8f4a-0eb3edcc2045?j=eyJ1Ijoiam9zaXAifQ.vsjhBZ3qKQPRsmtfvOuoYHIE7e7ZaHwbKLx QWJEessM).
Published July 29, 2023
About the Author
Joseph T. Shen, a recovered physician, Joseph T. Shen is the founder of Computational BioCybernetics & Lagrangian Mechanics Systems Engineering Technologies and Premier Heart, a company that produces the Multifunction CardioGram (MCG) technology. He was awarded the Medical and Science Achievement Award by the Women’s Heart Foundation in 2010 for his contributions to the development of MCG technology. Email: systemsmcg@icloud.com


