Burton M. Berkson, MD, PhD, and Francisco Calvo Riera, MD
This article was originally published in Clinical Oncology. 2021;6:1785.
Abstract
In this paper we describe the treatment of a 71-year-old female registered nurse (JA) diagnosed with Hepatocellular Carcinoma (HCC) in early October 2006.
The patient was a heavy smoker and drank alcohol on weekends. She contracted hepatitis C following a finger stick from a patient with the disease. Several years later she developed HCC.
Her doctor at a large medical center, after viewing her CT scans, told her that with so many tumors in her liver, only a liver transplant would offer some hope of survival. She was informed that without liver transplant surgery, given her diagnosis, any further treatment would, at best, be palliative. Mrs. JA refused liver transplant surgery.
The patient arrived at the Integrative Medical Center of New Mexico (IMCNM) in late October 2006. She appeared to be in very poor health, weak, and fatigued. An integrative program developed by one of the authors using intravenous alpha-lipoic acid, oral low dose naltrexone, and a healthy lifestyle program was initiated.1 After only a few treatments of IV ALA, her symptoms began to improve and her energy and outlook on life changed for the better. And she returned to work as a registered nurse.
From October 2006 to January 2009 the patient’s hepatocellular carcinoma was followed closely using PET/CT imaging. Her January 2009 PET scan demonstrated no residual increased glucose uptake in her liver. The patient had disappearance of the signs and symptoms of hepatocellular carcinoma 27 months following diagnosis with an integrative program essentially free of side effects.
This was one of the seven cases of serious cancer recoveries presented by one of the authors at The National Cancer Institute in 2012.2
Introduction
One of the most common malignancies in the world is hepatocellular carcinoma (HCC) often secondary to hepatitis C cirrhosis. There are about 700,000 new cases that are reported each year, with about 350,000 in China, and it is the third leading cause of cancer-related death worldwide. Only about 25% of hepatitis C patients benefited from conventional treatments before the use of the newer antiviral therapies.3,4
HCC is usually aggressive and far along in its development when most patients seek out treatment.
Therapies for advanced HCC include liver transplantation, surgery to remove the lesions, radiofrequency, ethanol injections, and transarterial embolization.5
Though treatment selections have improved recently, HCC survival rates are still poor, due to poor hepatic condition, comorbidities, or the presence of infiltrative and metastatic lesions. For patients with HCC, chemotherapy has been largely unsatisfactory, with less than 20% response rate, mainly because of the side effects of the treatment, especially on an already sick liver.
Therefore, it is imperative to develop novel therapeutics, particularly, if possible, without major side effects. In this paper, we call attention to how the use of IV ALA plus LDN and a healthy lifestyle may help reverse this life-threatening disease.
Mrs. JA, Hepatocellular Carcinoma Secondary to Hepatitis C
Mrs. JA, a 71-year-old widow and registered nurse presented to the Integrative Medical Center of New Mexico (IMCNM) in February of 2006 with a diagnosis of hepatitis C and HCC. She had had a CT scan with her internal medicine doctor, and it showed three low density lesions that were thought to be primary HCC.
She reported that she had gone through a great amount of stress due to the death of an adult child and the suicide of her husband. She was a smoker and drank alcohol during the week and more heavily on weekends. She was advised to immediately stop smoking and stop drinking alcohol.
Mrs. JA had a history of rheumatoid arthritis, hypothyroidism, arteriosclerotic vascular disease, situational stress and anxiety.
A few years prior to her visit to the IMCNM she suffered a needle stick from a hepatitis C patient and subsequently developed the HCC.
Her liver function at arrival at IMCNM showed an albumin level at 3.6 g/dl, platelet count at 120,000, prothrombin count at 12.4 seconds, and her ALT IU/l was 325 IU/l.
Her tumor marker for HCC, Alpha Fetoprotein (AFP), was 44 ng/ml (normal being less than 6 ng/ml).
Mrs. JA was advised to visit an oncologist and an interventional radiologist who might ablate her liver lesions. She said that her oncologist had given up on her, and she knew several hepatitis with C/ HCC patients who had undergone the conventional treatments and suffered serious side effects. And that those patients did not benefit from the therapy for very long.
She said that she was interested to undergo an integrative approach to this disease.
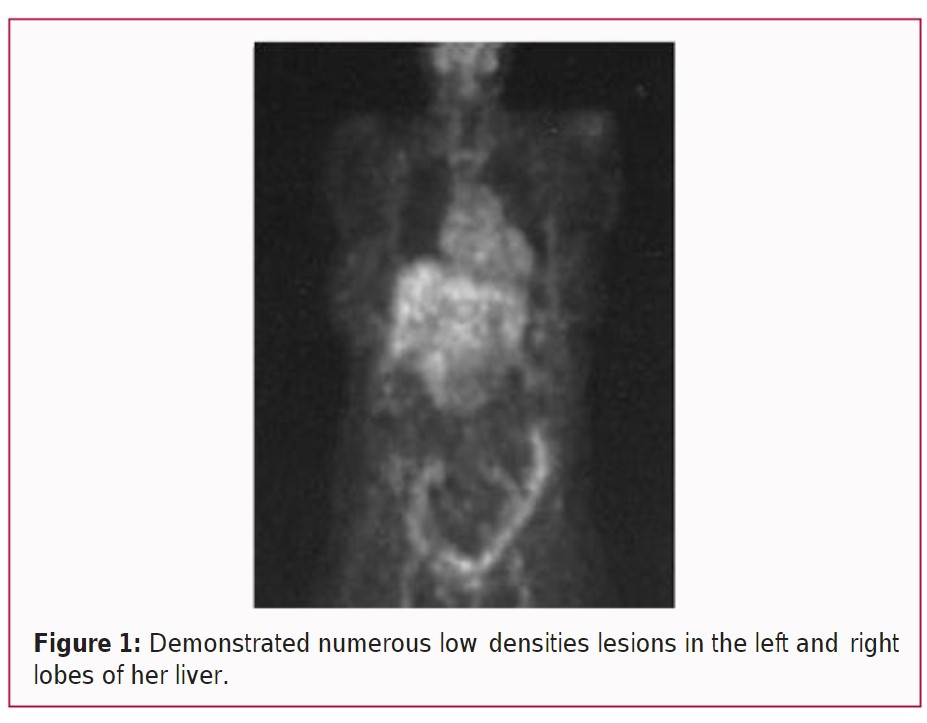
Mrs. JA was not adherent to the program at first and she continued to drink and smoke. A PET/CT scan of her abdomen was ordered on October 27th, 2006, and demonstrated numerous low densities lesions in the left and right lobes of her liver (Figure 1). Arterial vascular calcifications (ASVD) were also noted. The scan forced her to be more serious about the treatment.
The purpose of the INCNM therapeutic program was nutritional support, comfort, immune stimulation, and metabolic alteration of the malignant process. The hope was that her disease progression could be slowed and that her life might be prolonged. It was strongly recommended that Mrs. JA continue with her oncologist while on the integrative program; however, she refused.
In October 2006 Mrs. JA began her serious protocol of IV ALA 600 mg two or three times a week, low dose naltrexone 3 mg at bedtime, and the oral Triple Antioxidant Therapy (alpha lipoic acid 600 mg/d, selenium 200 mcg/d, and silymarin 1200 mg/d).1 On February 10th, 2007 she reported that she was beginning to feel much better.
Mrs. JA continued to improve and in a short time she said that she was feeling normal again. Mrs. JA continued the protocol until January 21st, 2009 when another PET/CT scan was done.
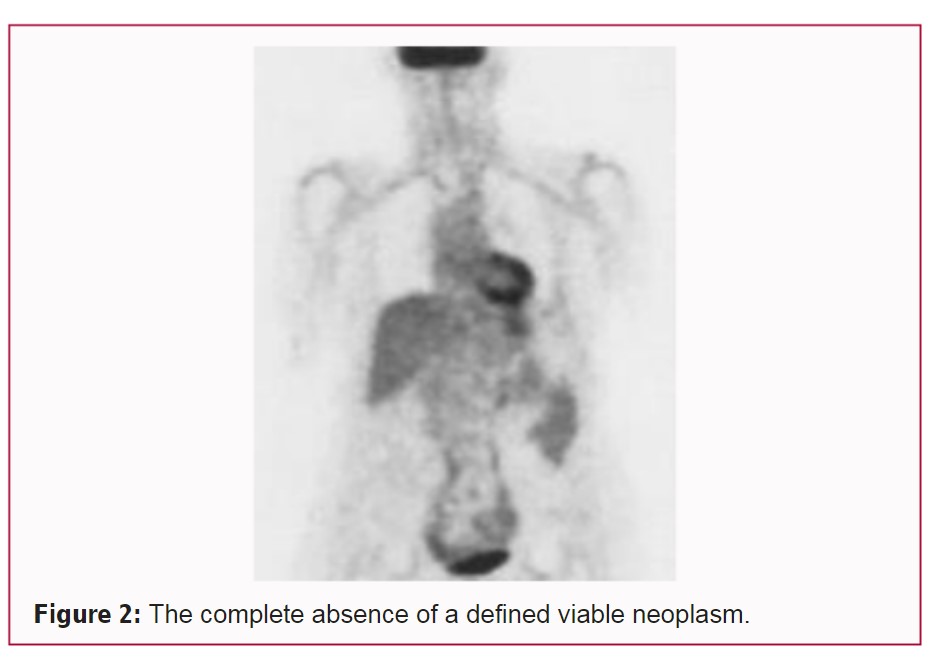
On that date the radiologist reported that there was no evidence of a viable neoplasm in her liver. Overall, he wrote, that compared to the prior FDG PET/CT study dated October 27th, 2006, there was complete absence of a defined viable neoplasm (Figure 2).
On October 25th, 2009, Mrs. JA’s platelet count was 159,000 (originally 120,000), her albumin was 3.9 gm/liter (originally (originally 3.6), her ALT was 111 IU/l (originally 325), and her prothrombin count was 11 seconds (originally 12.4).
It is interesting to note, that with a previous patient, Mr. JT, with a diagnosis of metastatic renal cell carcinoma to his left lung, even though he was feeling completely normal, it took almost four years for the lung metastasis to completely disappear.6
Following the favorable 2009 PET/CT scan, Mrs. JA discontinued treatment. She stopped the IV ALA therapy and the oral LDN and other supplements because she assumed that she was cured of her disease, even though she was told that the cancer could return.
Mrs. JA started smoking again, drinking alcohol, and continued working at a stressful nurse’s job. On November 22nd, 2010, she visited IMCNM again and said she was seeing an oncologist and a hepatologist. She said that she was feeling poorly and that her doctors told her that her cancer had returned.
She did not initiate the integrative therapies again but continued with her new doctors and started the conventional treatment for hepatocellular carcinoma. A short time later her daughter called our clinic and said that her mother had passed.
Materials and Methods
The intravenous alpha lipoic acid was obtained from McGuff’s Compounding Pharmacy in Santa Ana, California. Mrs. JA’s dosage was 600 mg in 100 cc bag of normal saline, infused in about 45 min, twice to three times a week.
Her low dose naltrexone was by prescription to Skip’s Pharmacy, 160 SW 12th Ave #102, Deerfield Beach, FL 33442. Her dosage was 3 mg at bedtime.
Her oral program consisted of the triple antioxidant therapy.1 Alpha lipoic acid 300 mg twice a day, selenium 200 mcg every day, and silymarin 300 mg four times a day from metabolic maintenance vitamins and supplements. Two B complex vitamins 50’s are added to the oral protocol because lipoic acid depletes several of the B complex vitamins.1
Mrs. JA was advised to stop smoking, avoid drinking alcohol, eat healthy organic food, and get some form of exercise.
Discussion and Review
In previous publications, the authors described the reversal of signs and symptoms of 4 patients with metastatic pancreatic cancer, one patient with a severe B cell lymphoma, and a man with stage four renal cell carcinoma treated among several modalities with alpha lipoic acid and low dose naltrexone.6–9
In the case of Mrs. JA only intravenous alpha lipoic acid, prescription low dose naltrexone, and the oral triple antioxidant protocol were used. She began feeling better after only one week of receiving the intravenous ALA and the LDN.
After 27 months her liver neoplasms had completely disappeared as demonstrated by the January 2009 PET/CT scan.
The prescribed key intravenous therapeutic ingredient was intravenous racemic alpha lipoic acid (ALA) 2 to 3 days a week after a meal (to prevent hypoglycemia).
Originally ALA was a prescription drug in Europe for the treatment of diabetes and its complications, and liver disease, including hepatocellular mushroom poisoning.10,11
Mrs. JA’s oral protocol included Low Dose Naltrexone (LDN) 3 mg at bedtime, and the oral Triple Antioxidant Therapy protocol with (1) racemic ALA 300 mg twice daily, (2) seleno-methionine 200 mcg once daily, (3) silymarin capsules of 300 mg four times daily (1200 mg) along with two professional-strength B 50 complex capsules a day, since ALA depletes several B complex vitamins. This protocol was originally described in a publication by one of the authors on the reversal of cirrhosis secondary to hepatitis C.1
The first successful large scale National Institutes of Health clinical trial using intravenous ALA (Thioctic acid) to reverse acute hepatic necrosis was completed by Bartter, Berkson and colleagues in 1980.11
Following these positive studies, Dr. Berkson was appointed the Food and Drug Administration Principal Investigator for ALA as a prescription drug for liver disease. Possibly, because the ALA could not be easily patented, no pharmaceutical company was interested in investing money to do expensive FDA clinical studies.
This IV ALA/LDN program had been used frequently at IMCNM with many patients and was previously reported in the scientific literature by the authors with favorable results in 4 cases of metastatic pancreatic cancer, one case of B cell lymphoma, the reversal of a metastatic renal cell carcinoma, and the reversal of signs and symptoms in three patients with hepatitis C cirrhosis.1,6-9
ALA has numerous important cellular activities, for instance it is a powerful antioxidant and heavy metal chelator, and the positive outcome in this case can, at least in part, be explained by its multiple mechanisms of action. ALA action to neutralize liver toxins was previously demonstrated by the successful treatment of a particularly powerful liver toxin, alfa-amanitin, which produces liver necrosis.11
It appears to us that several of its actions are most important in the case of cancer: Its profound effect in mitochondrial metabolism, its anti-inflammatory activity, and its epigenetic modifying activity.
Probably the most important is ALA’s participation as the major cofactor in the pyruvate dehydrogenase enzyme complex (PDH) and its action on the enzyme pyruvate dehydrogenase kinase (PDK).
Under normal oxygen concentrations, cells catabolize glucose anaerobically to pyruvate via glycolytic enzymes. Pyruvate is then taken up by the mitochondria. PDH is actually a complex consisting of three mitochondrial enzymes that sit in a very important crossroad: Cytoplasm and mitochondrion, glycolysis and the Krebs cycle, and anaerobic and aerobic energy metabolism. PDH converts pyruvate into Acetyl CoA, which is then further catabolized through the Krebs cycle or Tricarboxylic Acid Cycle (TCA), which transfers electrons to the respiratory chain.
Electron transport through this chain results in ATP production and completes the aerobic catabolism of glucose by donating electrons to oxygen.
ALA is the necessary cofactor for PDH. PDK phosphorylates and inhibits PDH,12 inhibiting pyruvate metabolism via the Krebs cycle. ALA, in turn, inhibits PDK activity,13,14 favoring the pyruvate flux into the Krebs cycle.
In hypoxic conditions, paradoxically, there is an increased oxidative stress derived from mitochondrial generation of Reactive Oxygen Species (ROS), with reduced cell survival.15 Under these conditions, the HIF (Hypoxia-Inducible Factor) not only favors anaerobic glycolysis by transcriptional activation of genes encoding glucose transporters and glycolytic enzymes, but also induces PDK gene expression and PDK protein level.15 By increasing PDK activity, HIF reduces the activity of PDH, the downstream Krebs cycle and its coupled electron transport activity, decreasing ROS production under anaerobic conditions, and thus preserving cell viability. In fact, it was found that under hypoxic conditions, HIF-1 null mouse embryo fibroblasts did not activate PDK and suffered apoptosis associated with a substantial rise in the level of Reactive Oxygen Species (ROS).15 We hypothesize that by having the opposite effect than HIF on PDK, that is, by inhibiting it, ALA promotes the flow of pyruvate through the Krebs cycle and electron transport chain, and under hypoxic conditions this promotes ROS production and impaired cell viability.
HCC is the second most hypoxic tumor (0.8% O2). As a reference, 5% is the physiological oxygen level in peripheral tissues).16 One of the most hypoxic tumors are pancreatic cancers (0.3% to 0.4% O2),16 from which there are several cases of ALA-induced remission.7,9 We wonder if the most hypoxic tumors are the ones more susceptible to ALA action, for the reason stated above.
In a related aspect, cancer cells very often show a metabolic peculiarity, the so-called Warburg effect, also called “aerobic glycolysis”: Cancer cells preferentially metabolize glucose and pyruvate into lactic acid, even in the presence of oxygen.17
This increase in glycolysis might favor the formation of amino acid and nucleotide precursors, important for a rapidly proliferating cell, whose importance might offset the disadvantage of a reduced ATP production.18,19
Seventy percent of viral and alcohol-related HCC show c-Myc oncogene over-expression, commonly due to its genomic amplification.20,21 Similarly, higher level of HIF-1 expression is often found and indicate a poorer prognosis in patients with HCC.22,23 Myc and HIF share several target genes of glucose metabolism: Both increase the expression of Glucose Transporter1 (GLUT1), hexokinase 2, Lactate Dehydrogenase A (LDHA), glyceraldehyde-3-phosphate dehydrogenase, and interestingly, both cooperatively activate PDK24, inhibiting aerobic respiration and contributing to the Warburg effect.25
McFate et al.26 have shown that inhibition of PDH activity contributes to the Warburg metabolic and malignant phenotype in human head and neck squamous cell carcinoma. This inhibition occurred by an enhanced expression of pyruvate dehydrogenase kinase (PDK). Knockdown of PDK restored pyruvate dehydrogenase (PDH) activity, reverted the Warburg metabolic phenotype, decreased invasiveness, and inhibited xenograft tumor growth in nude mice.
Recently, Lim et al.27 demonstrated that in renal cell carcinoma, most of the 10 tumor samples studied had an elevated PDK enzyme level, and a decreased PDH activity, when compared with patient-matched normal tissue.
Schell et al.28 furthered this idea by implying that the inhibition of the Warburg effect in colon cancer cells was associated with decreased cancer cell xenograft growth in nude mice.
Since ALA inhibits PDK and activates PDH, the metabolic peculiarity of cancer cells described by Warburg may be weakened, and it is likely that the overall cancer growth program may be altered.
In addition, ALA may discourage the growth of cancer cells by down-regulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB).29
NF-kB activation can produce proliferation, angiogenesis, mutagenesis, metastasis, and chemo-radio-resistance in cancer cells and leave them resistant to apoptosis.30
Patients with advanced cancer exhibit substantially raised markers of oxidative stress and a persistent inflammatory process in part due to NF-kB activation.30 ALA down regulates NF-kB, reducing these deleterious effects and slowing the uncontrolled growth of cancer cells.
Another potential antineoplastic action of ALA concerns its epigenetic activity. ALA can inhibit histone deacetylase (HDAC) activity in human tumor cells.31,32
Histone acetylation and deacetylation are important components in gene regulation. An active avenue of cancer research involves inhibitors of HDACs, with drugs such as vorinostat.
PDH, thought to be an exclusive mitochondrial enzyme, is also present and functional in the nucleus, probably translocated from the mitochondrion. Inhibition of nuclear PDH in isolated nuclei decreased the acetylation of histone lysine residues. This nuclear PDH has also lipoic acid as cofactor, so ALA provides a source for nuclear acetyl-CoA synthesis required for histone acetylation and epigenetic regulation.33
These epigenetic modifications give ALA the capacity of influencing, at a genetic level, tumor behavior and growth. In other words, cancer is not only a genetic disease but is also a metabolic disease. ALA seems to address both of these components.
Additionally, in 2020, Pibiri et al.34 reported that ALA induced apoptosis in HCC cells by stimulating endoplasmic reticulum (ER) stress and an unfolded protein (UPR) response by gene expression protein analysis. According to the authors, this finding provides a better understanding of the molecular mechanisms behind the anti-tumoral action of α-LA in hepatoma cells.
The other important agent in Mrs. JA’s treatment was Low Dose Naltrexone (LDN). It is an antagonist to Delta Kappa, Mu, and Opioid Growth Factor (OGF, also called Met-Enkephalin) receptors, and it does not have any narcotic effect. Naltrexone has a similar molecular structure to natural endorphins and at low doses (3 mg to 4.5 mg) binds to the OGF receptors on various cells for a short period of time, producing a temporal blockade that lasts about four to six hours, resulting in a rebound effect of an increase in endogenous endorphins.35 LDN interaction with the endogenous endorphin system is thought to reduce the proinflammatory cytokines and therefore a reduction in pain and to upregulate immunity.
Numerous publications have reported the use of LDN for the reversal of various cancers.2,6-9,36-39
Conclusion
In this report, we describe the treatment of a 71-year-old woman who was diagnosed with hepatocellular carcinoma in October of 2006. She was informed that given her diagnosis and refusal to consider liver transplant evaluation any further treatment would, at best, be palliative.
Mrs. JA arrived at the IMCNM in February of 2006, and she was weak and physically and emotionally exhausted. Her PET/CT scan of October 2006 demonstrated numerous tumors in her liver.
An integrative program developed by one of the authors (BB) using IV α-lipoic acid and low-dose naltrexone, and a healthy lifestyle program was initiated.
We think lipoic acid has a central role in the outcome of this case as it addresses two main cancer aspects: Metabolic (by its action on the PDH and PDK and the Warburg effect) and epigenetic (by its action on HDAC activity).
After a few treatments of IV ALA, Mrs. JA’s symptoms began to improve, and she started to put on weight. In addition, her energy and outlook improved, and she returned to work.
After 27 months her PET/CT scan of January 2009 demonstrated normal glucose uptake in her liver.
The patient demonstrated no signs or symptoms of hepatocellular carcinoma a full 27 months following diagnosis, with a sensible integrative program, which was essentially free of side effects. We feel that a treatment that is free of side effects is important in HCC, as the liver is often compromised by the disease and somehow intolerant to aggressive forms of treatments.
From the authors’ experience, however, when the treatment protocol is halted, in many cases, not all, the cancer growth resumes. Thus, the ALA/N (α-lipoic acid/low-dose naltrexone) protocol appears to induce tumor reduction/dormancy rather than cure the disease process.
Further multicenter studies are warranted to assess the success of this treatment regimen and long-term results on a wider population.
References
- Berkson BM. A conservative triple antioxidant approach to the treatment of hepatitis C. Combination of alpha lipoic acid (thioctic acid), silymarin, and selenium: Three case histories. Med Klin (Munich). 1999;94(suppl 3):84-9.
- Berkson BM. Alpha-lipoic acid plus low-dose naltrexone reviewed for cancer treatment. 2012. (Google Berkson, National Cancer Institute)
- Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004;127:5-16.
- Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010:29:4989-5005.
- Blum HE. Hepatocellular carcinoma: Therapy and prevention. World J Gastroenterol. 2005;11:7391-400.
- Berkson BM, Riera FC. The long-term survival of a patient with stage IV renal cell carcinoma following an integrative treatment approach including the intravenous α-lipoic acid/low-dose naltrexone protocol. Integr Cancer Ther. 2018;17(3):986-93.
- Berkson BM, Rubin DM, Berkson AJ. The long-term survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous α-lipoic acid/low-dose naltrexone protocol. Integr Cancer Ther. 2006;5:83-9.
- Berkson BM, Rubin D, Berkson AJ. Reversal of signs and symptoms of a B-cell lymphoma in a patient using only low dose naltrexone. Integr Cancer Ther. 2007;6(3):293-6.
- Berkson BM, Rubin D, Berkson A. Revisiting the ALA/N (alpha-lipoic acid/ lowdose naltrexone) protocol for people with metastatic and nonmetastatic 15 pancreatic cancer: A report of three new cases. Integr Cancer Ther. 2009;8(4):416-22.
- Ziegler D. Management of painful diabetic neuropathy: What is new or in the pipeline for 2007? Curr Diab Rep. 2007;7(6):409-15.
- Bartter, Berkson B, Gallelli J, Hiranaka P. Thioctic acid (alpha lipoic acid) in the treatment of poisoning with alpha-amanitin. Amanita toxins Poisoning, Verlag Gerhard. 1980;197-202.
- Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143- 51.
- Korotchkina LG, Patel MS. Pyruvate dehydrogenase complex regulation and lipoic acid. In: Patel MS, Packer L, editors. Lipoic Acid. Energy Production, Antioxidant Activity, and Health Effects. Boca Raton, FL: CRC Press; 2008:149-165.
- Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res. 2004;38:1083-92.
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177-85.
- McKeown SR. Defining normoxia, physoxia and hypoxia in tumours- implications for treatment response. Br J Radiol. 2014;87:20130676.
- Warburg O. On the origin of cancer cells. Science. 1956;123:309-14.
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11-20.
- Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029-33.
- Lin CP, Liu CR, Lee CN, Chan TS, Liu HE. Targeting c-Myc as a novel approach for hepatocellular carcinoma. World J Hepatol. 2010;2(1):16-20.
- Schlaeger C, Longerich T, Schiller C, Bewerunge P, Mehrabi A, Toedt G, et al. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511-20.
- Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int. 2014:409272.
- Chen C, Lou T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget. 2017;8:46691-703.
- Dang CV, Kim J, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8(1):51-6.
- Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39(3):231-4.
- McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700-8.
- Lim HY, Yip YM, Chiong E, Tiong HY, Halliwell B, Esuvaranathan K, et al. Metabolic signatures of renal cell carcinoma. Biochem Biophys Res Commun. 2015;460:938-43.
- Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400-13.
- Ying Z, Kampfrath T, Sun Q, Parthasarathy S, Rajagopalan S. Evidence that α-lipoic acid inhibits NF-κB activation independent of its antioxidant function. Inflamm Res. 2011;60:219-25.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-99.
- Van de Mark K, Chen JS, Steliou K, Perrine SP, Faller DV. Alpha-lipoic acid induces p27Kip-dependent cell cycle arrest in non-transformed cell lines and apoptosis in tumor cell lines. J Cell Physiol. 2003;194:325-40.
- Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin Cancer Biol. 2007;17:363-9.
- Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84-97.
- Pibiri M, Sulas P, Camboni T, Leoni VP, Simbula G. α-Lipoic acid induces endoplasmic reticulum stress-mediated apoptosis in hepatoma cells. Sci Rep. 2020;10:7139.
- https://www.ldnscience.org/
- Lissoni P, Malugani F, Bordin V, Conti A, Maestroni G, Tancini G. A new neuroimmunotherapeutic strategy of subcutaneous low-dose interleukin-2 plus the long-acting opioid antagonist naltrexone in metastatic cancer patients progressing on interleukin-2 alone. Neuro Endocrinol Lett. 2002;23:255-8.
- Lissoni P, Malugani F, Malysheva O, Kozlov V, Laudon M, Conti A, et al. Neuroimmunotherapy of untreatable metastatic solid tumors with subcutaneouslow-dose interleukin-2, melatonin and naltrexone, modulation of interleukin-2-induced antitumor immunity by blocking the opioid system. Neuro Endocrinol Lett. 2002;23:341-4.
- Zagon IS, Roesener CD, Verderame MF, Ohlsson-Wilhelm BM, Levin RJ, McLaughlin PJ. Opioid growth factor regulates the cell cycle of human neoplasias. Int J Oncol. 2000;17:1053-61.
- Hytrek SD, McLaughlin PJ, Lang CM, Zagon IS. Inhibition of human colon cancer by intermittent opioid receptor blockade with naltrexone. Cancer Lett. 1996;101:159-64.
Published June 17, 2023
About the Authors

Burton M. Berkson, MD, PhD, practices integrative medicine at the Integrative Medical Center of New Mexico (Las Cruses, NM), which he founded. Beyond his training as an MD, Dr. Berkson earned a Master of Science degree and a Ph.D. in biological sciences from the University of Illinois, Urbana. He has conducted research and taught at prestigious institutions, including the Max Planck Institute, the University of Illinois, and Rutgers University.
Dr. Berkson has served as an expert consultant on lipoic acid and hepatic poisoning for the CDC and former FDA lipoic acid principal investigator as a prescription drug. He is also the medical mycology expert for the State of New Mexico poison control centers. In 2007 and 2012, the National Cancer Institute invited Dr. Berkson to lecture on his innovative lipoic acid/low-dose naltrexone therapies, which correct mitochondrial dysfunction in various malignant diseases.
Dr. Berkson’s extensive research has resulted in numerous scientific papers, and he has authored and co-authored four books: The Alpha-Lipoic Acid Breakthrough (Random House-Crown, 1998), All About the B Vitamins (Avery, 1998), Syndrome X (John Wiley, 2001, with co-authors), and A User’s Guide to the B Vitamins. Contact: bberkson@nmsu.edu

Francisco Calvo Riera, MD, graduated in 1978 from the medical school in Valladolid, one of the oldest medical schools in Spain. He worked for three years at the medical center of Stanford University in California under the late Dr. Henry Kaplan, and later on he worked for three years at the Deutsches Krebs Forschung Zentrum (DKFZ, the German Cancer Research Center), and two more years at the “Professor Seelig Institute of Immunology” (Karlsruhe, Germany).
As he thinks that nutrition should be the solid foundation of all therapies, he studied the Gerson Therapy with Charlotte Gerson. He also has a master degree in nutrition by Cadiz University in Spain.
He has made learning visits and stages at the Riordan Clinic in Wichita, Kansas (one of the most experienced centers in integrative medicine and IV Vitamin C), USA, and has visited several times Dr Burt Berkson in Las Cruces, New Mexico, USA (the “father” of Alfa Lipoic Acid in his “Integrative Medical Center of New Mexico“), the Veritas Clinic in Lubbock, Texas, and Dr. Johann Lahodny Clinic in St Pölten, Austria, pioneer of the 10 pass, high dose ozone therapy.
In 2003, Dr. Calvo Riera founded the Autoimmune and Chronic Diseases Clinic in León, Spain.

