By Alan B. McDaniel, MD
Part 1 and Part 2 of this three-part article presented the complex physiology of thyroid function and discussed symptoms, diagnosis of hypothyroidism, and treatment.
At the First Follow-Up Visit
When your patient arrives feeling wonderful, simply test her blood levels after she’s been on the dose for at least two weeks. However, having conservatively brought her in before she reaches her calculated “maximum” replacement, you can expect to make small adjustments of 2.5 mcg T3 for 12.5 mcg T4—still replacing on a 5-to-1 basis. After her total dose has been moved to no less than 70% T4, not more than 30% T3, test her blood levels.
Blood tests are necessary to monitor therapy. The brief half-life of T3 makes its levels labile and testing mid-way between doses becomes even more important. I consult with patients who have been over-dosed because they were tested at trough and under-dosed because of being tested at three hours.
Ideal therapeutic values on combined T4 + T3 treatment are slightly different from normal reference intervals. When they feel best, their reports will not all be at the 50th centile! First, the patient is not healthy; she probably has deiodination problems (hence she needs T3). Secondly, her thyroid hormones are taken orally, not received intravenously; both hormones are altered during the hepatic first-pass. Also, doses every 12 hours, or even every 8 hours are not physiological. What blood levels “work” safely?
We’ve seen that patients seem to feel best with a tT3/RT3 ratio of 10 to 14. Since various local and national labs use different reference intervals for hormone assays, I will suggest relative values within those ranges, rather than numbers: The best freeT3 seems to be in the 75th to 90th centile while the “ideal” TSH, freeT4 and RT3 drop into the 1st quartile of “normal.”
At your second follow-up, when you review the lab reports on 70% T4 and T3 30%, the results may show your patient’s tT3/RT3 is still low. If so, make the adjustments you need to correct the ratio. Some people need less T4 and more T3. Others need either less T4 or a bit more T3. You will know after reviewing “all 5” tests to see which values are undesirable. Don’t worry—this is a skill that comes easily with practice.
Now: Review standard treatment goals.100 The first criterion is to restore tissue euthyroidism with resolution of symptoms and signs. On combined treatment, taking 70% T4 and 30% T3, Patient 2 seems to have fulfilled this. The second was also achieved: We maintained normal TSH and the most important physiological marker of thyroid function, the tT3/RT3 was restored to normal.77, 78 Thirdly, there is no evidence of thyrotoxicosis, clinical or biochemical.
Patient 2’s first and latest values are compared in Table 1. Her lab reports show that replacing T4 with T3 on a 5:1 equivalency maintained a stable TSH, confirming that for her, this substitution was satisfactory. As T3 was added to her regimen, blood T3 values predictably rose. With reduced T4, she produced less RT3: Her tT3/RT3 ratio was restored to normal. Her babies suggest this was efficacious.
The sharp-eyed observer will notice her latest freeT4 value is low. For several reasons, this does not worry me. First, the current reference intervals for freeT4 are skewed high. I say this from my experiences and consultations in 2009, when a national lab changed test platforms, raising the lower limit of their reference interval from 0.61 to the new 0.82. I followed many patients during this transition and the change was spurious.
Secondly, I observe that low RT3 is the best marker for physiologically low-T4. When freeT4 is maintained at or above 0.6 ng/dL, we predict the RT3 shall be 8 ng/dL or higher (I prefer “9”). Yes, I have erred in my learning curve and I’ve seen both freeT4 and RT3 go low; in that case, TSH rises and the patient feels just a bit “off”—but not badly, so long as her freeT3 is good. Once discovered, the problem can be corrected uneventfully.
Problems Particular to T3 Treatment
Patients need to cut the 5-mcg tablet in half. Recommend a good quality pill cutter with a cover; otherwise tablet halves will fly across the room. Until they develop skill at cutting pills, they may get a good half and the other as powder. Cut the pill when the dose is due; save the solid half for later and promptly take the powdered one. An alternative solution is to request compounded 2.5 mcg doses.
When prescribing compounded T3, never use the “SR delay-resin.” I have much experience with people taking this and I am wholly convinced: The microcrystalline methylcellulose or whatever they use does not delay absorption; it BLOCKS it by 50%–consistently. I am enough of a lab geek to feel certain of this.
Finally, generic T3 (liothyronine) is legally allowed to contain RT3—and it may. The FDA apparently considers RT3 inert. That this can occur in batches of T4 was noted in 2005.228 The contamination of generic T3 with RT3 was first identified by Denver’s Dr. Bob Menter, and it recurs periodically. Having no financial interest in any company, my experience shows the best generic is Sigma and the worst has changed its name repeatedly.
Therapeutics 202: Initiate Treatment for Hypothyroidism with “Natural” Thyroid
Desiccated thyroid extract, porcine USP (DTE or “natural thyroid”) is a valid option for treating hypothyroidism. Its use – originally from sheep’s glands – was first reported in 1892.229 DTE was still authoritatively endorsed as “highly satisfactory” in 1975224; and considering the recall statistics for “synthetic” T4 and T3 products, it has been relatively trouble-free. Despite criticisms and perhaps implausible attacks claiming natural thyroid carries a risk of prion-exposure,181 it is popular today and patients are more satisfied taking DTE than any other option.134
Most prescribers who treat with DTE do so because it contains T3.230 However, as I poll my patients for their choice of treatment, many have misconceptions about “natural vs. synthetic” that should be clarified.
What is better, natural or synthetic? Many people answer that “natural” is best, citing people like Patient 2, who do badly on T4. They say levothyroxine is synthetic, unnatural and therefore no good. Nope, that is not the explanation. To clear up confusion: Whether a hormone is from a “natural” or “synthetic” source is immaterial (unless you are allergic to the natural source, as some people are to pork).
Two issues are relevant. First: Is the hormone biologically identical, so it can perfectly fit its receptor and perform exactly as a human hormone should? Women have taken conjugated equine estrogens (CEE) for decades; these are hormones concentrated from pregnant mares’ urine (Premarin®) and wholly natural.231 They are not biologically identical: Some of the horse estrogens are so different from the human that they cannot be detected by laboratory tests. We cannot expect them to properly “fit” human estrogen receptors, either.
The second question is equally important: Is it the active hormone, or just a pre-hormone? Levothyroxine, on which Patient 2 had no joy, is biologically identical – the exact duplicate of human T4232—having been made-so (from what I cannot find) by chemical processing (i.e. “synthetic”). Being bio-identical, levothyroxine is 100% present and accounted-for on any lab test for T4. The problem with levothyroxine, as we’ve just seen, is not its mysterious origin or synthetic processing, but that it is a pre-hormone, not active.
The great merit of “natural thyroid” is that 20% of it is active T3. Proponents have claimed benefits also from the T2 it contains, but the quantity present is miniscule, so any effect is unlikely.233 We’ve just seen that Patient 2 needs to take 30% T3, not 20%; therefore, remember: DTE does give T3, but it may not give quite enough T3!
Which Patients Are Well-Suited for Treatment with DTE?
I believe anyone who asks for “natural” thyroid is an excellent candidate. There are reasons it would be an inappropriate choice, especially allergy to pork. It is, of course, decidedly not kosher or vegan; and I leave the final decision up to the patient and her rabbi, imam, or nutritionist—who usually endorse it.
The pre-treatment assessment of “all-5 hormones” importantly contributes to this decision. While most studies report hypothyroidism is associated with increased tT3/ RT3 as the scarce T4 is optimally utilized, some patients have low tT3/ RT3 even in their hypothyroid state.211 It is very unlikely that T4-only treatment would correct this imbalance, and recommending DTE is appropriate.
DTE Pre-Treatment Considerations and Informed-Consent Counseling
Prescribing DTE is similar to properly giving T4. Your informed consent talk will review the therapeutic goals for treatment; dose-response curve and the risks, complications and side-effects already discussed. Immune hypersensitivity to the natural product should be mentioned; it is more common than sensitivity to levothyroxine. Caution women about increased fertility: Accidents cause people!
Add this information too: Most other practitioners were trained to believe “natural” thyroid is unreliable and even dangerous. Your patient must know in advance that she may have to defend her use of it and refuse to let a well-intended provider replace it with “safe and reliable” levothyroxine.
Before treating hypothyroidism with desiccated thyroid extract, estimate the patient’s likely maximum dose. If there is no concern about autonomous function, a healthy adult’s “maximum” dose is about 1 mg DTE thyroid per pound of lean body weight per day (2.2 mg/ Kg). So, a fit, active woman of 120 lbs. may be able to take 120 mg DTE daily. I think the use of DTE seems sufficiently archaic without quantitating it in “grains.” If anybody needs to know, one grain is about 60 mg.
As it is for levothyroxine, the optimal DTE dose seems most closely-related to lean body mass. If my patient was once a 120 lb. athlete but she is now exhausted and weighs 200 lbs., her maximum dose of DTE should not be 200 mg/day. Adipose won’t require as much thyroid hormone as muscle …but she has built more muscle from carrying the extra weight! Here again, be conservative …and gain the experience to use “Kentucky windage.”
We prescribe DTE because it has T3, but its half-life is brief – just six hours. Therefore, it is imperative to divide the dose of DTE at least every 12 hours, and sometimes, like Osler, every eight hours. Tell patients to set a reminder alarm on their cell phone and to carry a few doses at all times. It is a good rule to take DTE no less than four hours before bedtime, lest peak-levels of T3 keep them awake. Taken every eight hours, the bedtime dose causes no sleep disruption and usually improves its quality!
Begin Treatment with DTE: Dose and Timing
Adult patients start taking 15 mg DTE every twelve hours. These tablets are the smallest made and the dose can be adjusted to an excellent “fit.” NEVER start a “full dose” of DTE; it is too risky. Hypothyroidism increases the population of nuclear thyroid-hormone receptors.234,235,236 This large cadre of “hungry” receptors is very sensitive to replacement T3—especially to overly exuberant initial doses or too-rapid escalation.
Gradually increase this dose once-weekly, as tolerated; patients must understand this concept before they take any thyroid preparation containing T3. As with T3, DTE pre-treatment counseling about caffeine use, symptoms of low estrogen, and reactive hypoglycemia from a poor diet is more important than for levothyroxine. Complaints are more frequent because DTE is more effective.
Dose Escalation
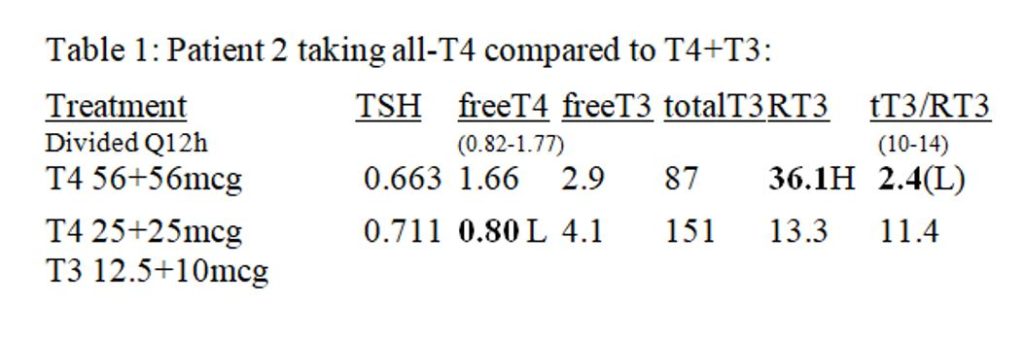
Give written instructions for patients to build their DTE dose once-weekly, as tolerated. Again, the dose-response curve (Figure 2) is a useful “visual-aid.” Direct her escalation to less than the calculated “daily maximum” and follow-up promptly as a safety measure. For example, plan to give a 5’ 5” tall young woman weighing 130 lbs. no more than 120 mg DTE /day before reviewing her progress. Conservative instructions are shown in Figure 7.
Figure 7: Typical DTE thyroid dose escalation for 130 lb. patient
Make an appointment for the end of the final week of escalation, to review her responses during the process and inventory the remaining symptoms. Remain alert for complaints and issues during escalation. Again, these are somewhat more troublesome with DTE than T4, due to the T3 content. Usually, all goes smoothly.
As always, dose escalation is conditional, depending on results of the last change: Better, same, or worse? People often feel “unsettled” for a few days after a dose change, so wait a week before deciding. Your patient should call if she unexpectedly feels worse.
Most patients on DTE feel incrementally better as the dose is increased. Think of a person climbing a ladder—each step noticeably takes him closer to the top. A few people simply feel no better until about five days after the precisely correct dose is reached; then suddenly all is great. I suspect this is when the tT3/ RT3 ratio finally “pops” into place. I think of them as “vending machine” people; if it takes 40 cents to get a pack of gum, nothing happens until you’ve put in the quarter, the dime and that last nickel.
The First Clinical Follow-Up on DTE
If your patient feels “100% well,” which often happens, the path is certain: Check blood levels. Repeat the blood tests in a few months if all is still well and again after another six months. After that, annual follow-up labs are mandatory—sooner if symptoms change.
When symptoms indicate her therapeutic response is incomplete, ask more questions: Do the symptoms worsen shortly before the next dose is due? This suggests under-treatment. However, if she feels noticeably worse 2-3 hours after her dose, suspect an over-dose or allergy to pork…or caffeine interaction etc. Consider the adrenals, low estrogen, and a bad diet if not all is well.
If she seems still under-dosed, that’s actually the plan, right? Build her dose by smaller steps: 15 mg increments weekly. For the hypothetical 130 lb. woman above, give first 60 mg Q 12h and the next week, maybe as much as 75 mg and 60 mg. I’d go no higher without the assurance of the laboratory that she needs more. Any time you feel “bewildered,” use the laboratory for a navigational fix.
The first laboratory follow-up is performed the same as always: At pharmacological equilibrium, the blood sample is drawn mid-way between regularly timed doses. Test “all-5”; the “ideal” results will be the same as those of combined T4 and T3 treatment.
Problems with “Natural” Thyroid Treatment
Allergy to pork has been mentioned and uniquely, “natural” thyroid also contains elements of the thyroid glands from which it has been extracted. Rarely, DTE has significantly intensified thyroid inflammation in a patient with Hashimoto’s, apparently from adding reactive-antigens to the already hyper-immune gland. This problem resolves once the dose is raised sufficiently to suppress TSH—but it gives everyone some concern initially. I add this to my IC-talk prior to treating with DTE, as a possible adverse event and relative-contraindication.
Unfortunately, TSH is often suppressed by the DTE dose that gives the best symptom-relief. My best explanation is that the patient’s thyroid gland continues making too much T4 (converted to RT3) until it is suppressed by DTE’s richer mix of T3. Simply put, more than 80% T4 is often too much.
As long as blood levels of the thyroid hormones are normal, low TSH is no physiological problem. Low TSH does not damage bones – high T4 does!237 Low TSH doesn’t affect the heart; high T3 does.238 However, some practitioners incorrectly assume low TSH means that you’ve made the patient hyperthyroid. So, your TSH-suppressed patient must understand this to defend her treatment from “good intentions.”
There have been shortages of “natural” thyroid. Since the “great Armour® famine of 2009,” we’ve had recalls of NP Thyroid® and Nature-Throid®. Rest assured; it is simple to convert from DTE to bio-identical synthetic. The PDR tells us 60 mg of Armour® delivers 38 mcg T4 and 9 mcg T3: Just do the math. While many patients had to switch from DTE to T4 and T3 in 2009, not all switched back when the “natural” was again available.
Trouble-Shooting Other Providers’ DTE-Patients
Expect to see other practitioners’ patients doing badly on once-daily DTE. As you now know, dividing doses is necessary because the 20% T3 in DTE has a short half-life and the 80% T4 in a single bolus can and will be excessively converted to RT3. Patient 3 was one of these.
She arrived taking DTE 90 mg every morning. She was tested as close to mid-dose as possible, just before the lab closed at 5PM and her values were: TSH= 0.54; fT4= 0.77; RT3= 320 and tT3/ RT3= 4.3. Her dose was divided Q 12H and because of her body size, increased to 60 mg AM and 45 mg PM. Her next blood tests were drawn six hours after the AM dose: TSH= 0.42; fT4= 1.28; RT3= 256 and tT3/RT3= 8.7 (better but she needed less T4).
Confusion can arise also from “pre-analytical” decisions to test at what might be called “the wrong time.”
Patient 4 consulted me because she was unhappy taking DTE 60 mg every AM, which had been followed with tests drawn 3 to 4 hours after her dose. Because of high freeT3= 4.5 H at this time (“peak”), she was told this was her maximum allowable dose.
After discussion, Patient 4 divided her DTE to 30 mg Q 12h. Predictably, she reported her energy was “more consistent, but only moderate.” She had less energy in the AM and early afternoon but more energy in the late afternoon and evening. When tests were drawn at mid-dose, 6 hrs. after her AM dose, her freeT3 was just 3.1 and tT3/RT3 was only 4.2 (L). No, she was not on her maximum dose; her story is continued below.
Patient 5 came from the same provider, with the same set-up: Once-daily DTE 90 mg was tested four hours after her dose and her high freeT3= 4.6H implied she was maximally treated. On my recommendation, she was re-tested on 45 mg DTE Q 12h at six hours after the AM dose. Her freeT3 on the same daily dose was now only 2.8.
Other patients have simply not been monitored for therapeutic levels. Patient 6 was treated with DTE by an open-minded doctor who adjusted doses solely according to her clinical response. As the dose was increased, she felt better in some ways but worse in others. When she came to see me, the 221 lb. young woman was taking 240 mg DTE daily. The doses were divided, 120 mg twice-daily, but she had to take the second dose only five hours after the first – otherwise, she couldn’t fall asleep at night.
On my request, Patient 6 endured taking DTE 120 mg every 12 hours for long enough to be accurately tested at mid-dose. She was therapeutically hyperthyroid. She had an “acceptable” tT3/RT3 because very high T3 levels balanced her excessive RT3: TSH=0.007L; fT4=1.43, fT3=7.0H; tT3= 31.6H, RT3= 30.7 and tT3/RT3= 10.3. We are emphatically reminded that symptoms alone cannot be trusted to guide therapy.
Therapeutics 301: Use “Natural” Thyroid to Correct Failed T4-Treatment
When T4-only treatment fails, replacing some T4 with T3 can succeed and is now acceptable to most of our colleagues. It is also possible to replace T4 with DTE. Patients ask for this, and it is often very satisfactory. Several tactics can be successful.
First, one can use the rough equivalent 25 mcg T4 ≈ 15 mg DTE. The steps are simple: First, prescribe 25 mcg T4 tablets and 15mg DTE tablets to make dose adjustments easier. Next, divide your patient’s daily T4 dose to Q 12 hours for a week, allowing her to get into equilibrium—and then start replacing T4 with DTE. Because T3 gives “more energy” than T4, start DTE first with the AM dose.
Caution: This formula makes 100 mcg T4 the equivalent of 60 mg DTE—which is rather a low dose and may not be sufficient. Using this semi-equivalency, patients often need to continue building their DTE dose after the T4 has been wholly replaced. True confessions: Patients have reported feeling great after replacing T4 with DTE; their tT3/ RT3 was about 11 and to my surprise, so was the TSH—it’s that learning curve again!
Secondly, one can think of DTE in terms of its constituent T4 and T3, rather than as milligrams or grains. As above, each 60 mg (“1 grain”) DTE thyroid contains 38 mcg T4 and 9mcg T3.239 When you examine your patient’s “all-5” lab values on her unsuccessful T4 treatment, you’ll see whether she needs only some added T3 or a reduced amount of T4 plus T3. Then, you are able to substitute-in the DTE with scientific finesse. But it doesn’t always work, because DTE offers no less than 80% T4.
Therapeutics 302: Correct the Failure of DTE Thyroid Treatment
Over the years during which I logged hundreds of patients for whom T4-only treatment gave poor results, I also recorded many scores of patients who had suboptimal results from DTE thyroid. Here again, the “post-analytical analysis” of lab reports is so important! As with T4, incorrect dosing occurs—either too much or too little, as the patients above demonstrate—but by far the most frequent problem was dysfunctional deiodination of T4, indicated by low tT3/RT3.
The DTE content of 20% T3 and 80% T4 would not be a “rich” enough mixture for Patient 2, who needed 30% T3 and 70% T4—as validated by her outcomes, both laboratory and obstetrical. Patient 7 also needs more than the 20/80 in DTE: This 68 year-old man felt better after replacing T4 with DTE but even so, his tests on “natural” 60 mg Q 12 hrs., taken six hours after his AM dose showed a low tT3/ RT3 ratio: TSH=0.01L; fT4=1.0, fT3=3.6; tT3=83, RT3=20 and tT3/RT3=4.2(L).
Coach, review “all 5-players” on Patient 7’s report: Low TSH indicates his total dose is plenty and that all the thyroid hormones in his blood come from DTE treatment. Excessive T4 intake is indicated by two things: A. His freeT4 is higher than my “goal” and B. The tT3/ RT3 is very poor due to robust RT3. Hence, DTE gives him too much T4. He also may need more T3: His freeT3 could be higher and totalT3 clearly looks sub-par. We agreed to adjust his intake.
DTE failed: It gave Patient 7 too much T4.
During informed-consent talk, Patient 7 was given several options. The first is a commonly used method, to replace DTE 15 mg with T3 5 mcg (each is the smallest tablet of its kind). This inexact exchange is based upon their comparable effects on a healthy person’s TSH-production. However, healthy people activate T4 normally and Patient 7 cannot.
The second option is to do the math: Upon calculating the amounts of T4 and T3 in DTE 15 mg, we find this exchange would undesirably increase T3 as it reduces his T4. Let me “show my work”: We’ve seen that 60 mg DTE contains T4 38 mcg and T3 9 mcg;239 thus, we calculate 15 mg DTE contains 9.5 mcg T4 and 2.25 mcg T3.
To achieve our goal of reducing Patient 7’s T4 dose, we should replace DTE 15 mg (9.5 mcg T4 and 2.25 mcg T3) with only 2.5 mcg T3 (½ tablet). I have come to rely on simple mathematics—and to regard DTE as a vehicle for delivering an 80% T4/20% T3 mixture.
An engineer, Patient 7 surprised me and chose a third option: He wanted to stop DTE altogether and to take T4 and T3 individually—so we did. The required substitution was simply calculated: From DTE 60 mg Q 12 hrs., Patient 7 daily received 76 mcg T4 and 18 mcg T3. We replaced it with levothyroxine (T4) 75 mcg and liothyronine (T3) 20 mcg daily (divided Q 12hrs.).
Because “the Coach” predicted Patient 7 was taking too much T4, his T3 dose was maintained at 10 mcg Q 12 hrs. while the T4 was sequentially reduced once a week: It dropped from 37.5 mcg Q 12…to 25 mcg Q 12…to only 12.5 mcg Q 12 hours. This T4 dose—now diminished by 50 mcg daily—felt best to him. Admittedly, we dropped below my 70/30 “guideline”: The proportion of T4 had fallen to only 56% T4, with 44% T3. He was tested.
“Mid-dose” blood tests (on T4 12.5 Q 12h and T3 10 Q 12h) verified that for him, “less is more”: TSH=1.80; fT4=0.68 L; fT3=3.7; tT3=115, RT3=9.1 and tT3/RT3=12.6. His TSH had become normal. As predicted, he needed about the same T3 he had gotten from DTE. Although I was tempted to increase his T4 a bit, this dose was maintained…and his subsequent fT4 values rose to 0.88 and 0.82, “normal.”
DTE thyroid failed: Patient 4 needs less T4 and more T3.
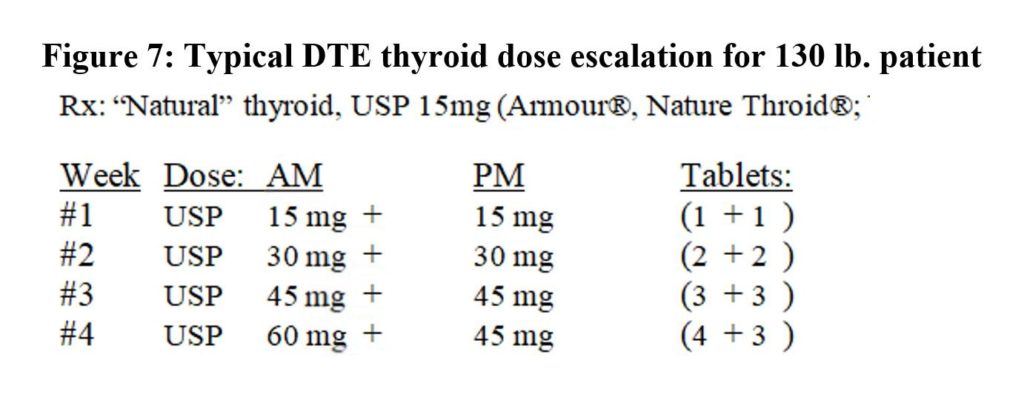
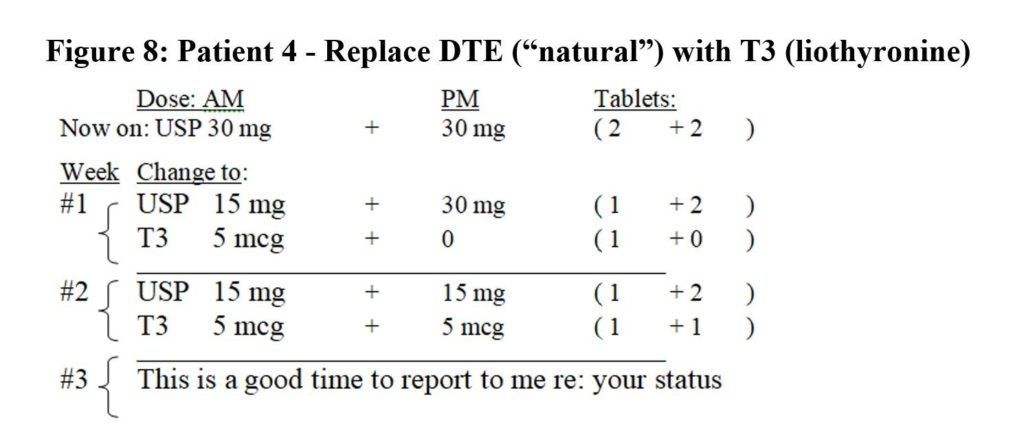
Patient 4 had insufficient energy and undesirable mid-dose blood values on DTE 30 mg Q 12 hrs.: TSH=1.93; fT4=1.16, fT3=3.1; tT3=85, RT3=20.1 and tT3/RT3=4.2 (L). She and I reviewed her options. We agreed she would take a little less T4 and increase her T3 by replacing DTE 15 mg (9.5 mcg T4 and 2.25 mcg T3) with 5 mcg T3 to reach DTE 15 mg Q 12 and T3 5 mcg Q 12: A 50:50 mix. This was done in two small steps (Figure 8).
Her new therapeutic values at mid-dose were better: TSH= 1.97; fT4= 0.87; fT3= 3.5; tT3=121, RT3=12.1 and tT3/RT3=10. She later divided her doses to take the same amounts divided every eight hours, and her tT3/ RT3 ratio rose to 12.5 (DTE 0+15+15 and T3 5+2.5+2.5). She has referred five people to my practice in the last year.
Figure 8: Patient 4 – Replace DTE (“natural”) with T3 (liothyronine)
Patients may need only more T3. When the laboratory shows your patient has enough T4 and “the Coach” says they need T3, just add it—cautiously!
Therapeutics 401: Challenges in Hypothyroidism
Athyreotic patients have no thyroid gland. They are the least tolerant of once-daily levothyroxine, for they make no T3 and have no endogenous safety-net! On arrival to your office, they collectively are on the widest variety of unusual dosing strategies. Be of good cheer: You now have all the skills to delight them with the results of your work. Keep them on the same average daily T4 dose they arrived taking (add up their weekly dose and divide by 7) but divide it evenly every 12 hours. Then, after two weeks, test “all-5” at mid-dose and see what they need.
They will need T3, of course. Let your well-honed “Coach” tell you how to modify their T4 during the process of escalating T3. Every eight hour-dosing may ultimately be best. These will actually be easy and rewarding patients: Their thyroid gland is “gone” but that means its function is stable. Once they are “right,” they’ll probably be low maintenance.
Unfortunately, NOT all hypothyroid patients are stable. They can offer an uncomfortably “moving target.” In these cases, residual thyroid function keeps changing, and the treating practitioner must stay alert and adaptable.
Progressive loss of thyroid function occurs and not uncommonly. When we treat hypothyroidism due to Hashimoto’s disease and maintain TSH=2.0, it is likely the patient’s thyroid gland is adding some hormones to the mix. If thyroiditis continues to destroy the gland, it can add less hormone and TSH will rise, indicating the patient needs a larger dose. Follow blood tests annually—and sooner if symptoms change.
Autonomous function mocks our efforts to calculate “maximum” doses. When any portion of the patient’s residual thyroid function carries-on without appropriate HP-T feedback, it can make treatment difficult. The simplest of these is Hashitoxicosis, which can be present to some degree in up to 10% of cases.240 The destructive autoimmune process causes the uncontrolled release of hormones stored in the colloid, resulting in transient hyperthyroidism or unexpectedly high hormone levels.241 Because it usually resolves in two months, it should not interfere with treatment for hypothyroidism in advanced cases – unless there is an ongoing, “smoldering” process that is unmasked when TSH-suppression is a therapeutic goal.
Graves’ disease and “switching” offers the most complex autonomous function we are likely to encounter. Graves’ is caused by the production of TSH-receptor stimulating antibodies, which cause hyperthyroidism. It can also feature TSH-receptor blocking-antibodies, which produce hypothyroidism (“atrophic Hashimoto’s”). Finally, most Graves’ cases also test positively for the (probably cytotoxic) anti-TPO and anti-Tg antibodies of Hashimoto’s.242 The diseased thyroid can flip from hyper- to hypothyroidism at a whim – and may then become hyperthyroid again.243,244,245
Patient 8, a woman age 34, gained 40 lbs. in three months in 1996 and then quickly lost it again. Her endocrinologist correctly diagnosed Graves’ disease (TSH< 0.03 L, tT4= 14.8 H and 123I* uptake= 67% H) and put her on methimazole; she promptly regained 30 lbs. and quit taking the drug.
Seven years later (March 2003), she consulted me for symptoms of hypothyroidism; tests showed her TSH was 2.04 and her tT3/ RT3= 4.0 (L). I next heard from her a year later, 3/2004, when unemployed and tested at a free clinic, her TSH= 51.5 H. She took replacement thyroid hormone for a while and again became lost to follow-up.
Three years later, in 12/2006, she was working, insured and had marked hyperthyroidism. Taking no thyroid treatment, her labs showed: TSH=0.013 L; fT4=2.29 H, fT3= 6.3 H; tT3= 268 H, RT3=51.3 H. tT3/RT3= 5.2 (L). Validating the diagnosis of Graves, tests showed: TPO-Ab=72 H, Tg-Ab=161 H, TR-Ab= 36.6 H; 123I* Uptake= 48% H.
Despite the high hormone levels, her symptoms mostly suggested hypothyroidism; perhaps from the very high and in this case adaptive levels of RT3. Thyroid ablation was indicated. Reviewing this case, it is easier to understand why so many US physicians promptly ablate Graves’ hyperthyroidism with 131I*.246
During pregnancy, thyroid binding proteins are remarkably increased. For this reason, up to 80% of hypothyroid women who become pregnant will need larger replacement doses—increased by as much as 45%.247 American Thyroid Association (ATA) pregnancy-guidelines are available as a free download.248 They recommend testing as soon as the pregnancy is diagnosed and following blood levels every four weeks thereafter.
Prompt testing is needed because hypothyroidism is associated with first trimester miscarriage, as Patient 2 showed us. In fact, ATA publications even state there is a “clear association between thyroid antibodies and spontaneous pregnancy loss.”248 Thus, their guidelines are exacting, to protect the child and safeguard its development: The upper limit of TSH should never exceed 4.0 (others say “2”) and freeT4 must be kept above the 10th centile.
Do not feel dismayed when a pregnant patient’s totalT3 and RT3 values zoom up: It is because of the greatly increased thyroid binding proteins! Fortunately, the binding constants of T3 and RT3 are very similar, and their ratio will continue to be valid for comparison. After delivery, the hormone doses can be reduced. The literature and experience agree that new mothers can reduce their doses about six weeks postpartum.
Gestational transient thyrotoxicosis is an unusual disorder that is not immune-mediated. Hyperthyroidism develops because the pregnancy hormone, human chorionic gonadotropin can too closely resemble TSH. It cross-reacts with the TSH-receptor and over-stimulates thyroid gland.249 Fortunately uncommon, it could make your patient’s pregnancy even more challenging, depending on the amount of her residual thyroid function.
Oral contraceptive pills and ovarian hormone replacement also stimulate the liver to make greater amounts of thyroid binding-proteins. Thus, when hypothyroid women start these treatments, they too may need increased thyroid replacement and should be tested.250,251,252 Conversely, when these treatments are discontinued, their thyroid levels should again be checked.
Therapeutics 402 (Senior Honors): Subclinical Hypothyroidism
Subclinical hypothyroidism is a conundrum for experts. The diagnosis describes a mild case of hypothyroidism, in which patients theoretically have no symptoms (this criterion is now generally disregarded); have elevated TSH to less than 10 µIU/mL, and freeT4 within normal limits.37,253,254
Because no clear benefit has been proven in placebo-controlled T4 treatment-trials lasting up to 18 months, committees of experts consistently state these patients should not be treated,255 with the exceptions of women hoping to become pregnant and possibly of adults younger than 30.256 Some endorse giving T4 to markedly symptomatic “subclinical” patients—as a trial, which must be stopped if there is no clear benefit.253
Pediatricians “consider it reasonable to initiate treatment to avoid any potential risk of negative impact on growth and development.”100 Doing so, they recommend therapeutic T4 levels in the mid to upper half of the reference range and the TSH “optimally between 0.5 and 2.0 µIU/L.”
Subclinical hypothyroidism creates controversy because of this paradox: Many studies have shown that these “subclinical” patients without treatment will, in the long term, have worse rates of dysfunction and death compared to healthy controls.257,258,259,260 However, we’ve seen that many other studies can show no benefit from T4 treatment until TSH rises above 10 or freeT4 becomes sub-normal.37, 254, 259,261
So, even though people with mildly abnormal tests have significantly worse outcomes, T4-treatment should be given to only markedly ill patients, else it will not help. Another way to state this is that levothyroxine is so ineffective that it can help only severely deficient patients.
None of the authoritative reviews or a meta-analysis262 have examined or mentioned T3 treatment arms. In fact, the definition and treatment criteria for subclinical hypothyroidism do not account for T3, which we know correlates with symptoms,144—much less do they consider RT3.255
We are returning to an age of combined T4 and T3 treatment. We now can ask: Will adding T3 to T4—and achieving the “desirable” blood levels I have suggested above—succeed in delivering the benefit these “subclinical” patients fail to receive from T4? From my patients’ results, I answer this question with an unqualified “yes – it can.”
It has been 21 years since the first good evidence that T4 + T3 combination treatment can be superior to T4 alone was published.263 Let’s hope we do not have to wait equally long for the proof of what seems so likely to be the case, that subclinical hypothyroidism can respond better to T4 + T3.
Post-Graduate Seminar: Resistance to thyroid hormone (RTH)
We have just seen that subclinical hypothyroidism is a condition without any clinical symptom (so said) that is revealed only by the laboratory. For years, some physicians have believed the converse must exist. They see patients with many thyroid symptoms and signs, but the standard tests (TSH, freeT4) are negative: One could call it “Sub-laboratory hypothyroidism.”
Some physicians offer thyroid hormone supplementation to these patients. When they are rewarded by clinical improvement, they continue the treatment – despite some risk to themselves.264 Thirty years ago, 12% of patients taking thyroid hormone were thought to do so “inappropriately,” by prevailing guidelines.111 These patients usually took “natural” thyroid, not levothyroxine, for a variety of indications and most continued taking it.
What do these risk-tolerant physicians believe they are treating? In browsing the internet or standing by the coffee urn at medical meetings, one encounters the phrase “resistance to thyroid hormone.” This does indeed occur. There are three hereditary types; the causes are different and presentations distinctive.
Dysfunctional Thyroid Hormone-Receptors
The first type of resistance to thyroid hormone (RTH) is caused by mutated genomic thyroid hormone-receptors,265 known as Refetoff syndrome. There are two major types of thyroid receptors, alpha and beta, each of which can be affected by loss-of-function mutations. The consequences can be mild to severe, depending on the degree of impairment.266
There are about 1,000 cases world-wide, many more beta than alpha. Mutations of THRB, the gene encoding thyroid hormone receptor-beta are more common and less severe. The clinical features include elevated levels of both TSH and thyroid hormones (since the receptor lacks sensitivity) and a goiter. Treatment with T4 can help symptomatic patients but should not be offered to all affected.267 Laboratory values will not return to “normal,”268 and all surgically removed goiters have recurred.267
Patients with a mutated THRA gene encoding thyroid hormone receptor-alpha were first identified in 2012.269 The effects of this mutated, dysfunctional receptor range from delayed puberty to more severe dysmorphic features.270 Their laboratory profile is unusual: “Standard” tests are normal but unusually, RT3 is low and consistently, the T4/T3 ratio is also sub-normal. While higher T3 concentrations can reverse receptor dysfunction in-vitro, T4 has been employed for clinical treatment and it may help—or not.266
Disorders of Thyroid Hormone Transporters
Lipophilic thyroid hormones enter cells via trans-membrane transporters.271 In this second type of RTH (“Visser syndrome”), defective MCT8 transporters cause severe neurodevelopmental disabilities, which are usually identified in infancy. These children also have high serum total and free T3 and low RT3 concentrations. T4 is reduced in most cases and TSH levels can be slightly elevated but rarely above 6 mU/L.267 Symptomatic problems with the other major transporter, OATP1C1 also have been described.272 Treatment efforts are largely unsuccessful for both types.
Dysfunctional Deiodinase Enzymes
This third type has two subsets also. This type of resistance to thyroid hormone is caused by dysfunctional deiodination of T4. Refetoff included hereditary thyroid hormone metabolism defect in his recent review of insensitivity to thyroid hormone.267
In the first subset, nine families (it’s rare!) have an inherited defect of selenoprotein synthesis, resulting in severely dysfunctional deiodinase enzymes and clinical hypothyroidism. The children have growth delay, mental and neuromuscular defects. The only known adult (male) had fatigue, muscle weakness, severe Raynaud’s, vertigo and hearing loss; skin UV-sensitivity and infertility.267 No patient had an enlarged thyroid.
Laboratory tests show low T3, high T4, high RT3 and normal or slightly elevated TSH. These lab values resemble acute ESS/NTI (note the authors), from which it is distinguished by elevated TSH and by a general medical evaluation.267 For the few patients treated, T3 was successful: It bypassed the hereditary defect, suppressed TSH as it does in normal people and improved the children’s delayed linear growth.273,274
DIO2 Mutation: In contrast to the severe dysfunction caused by the above selenoprotein synthesis-defect, the mutated DIO2 carried by 16% of tested Britons causes a mild problem.136 The mutant type 2-DI works well enough for normal childhood growth and development. Its dysfunction may not be detected, as long as the carrier’s thyroid normally makes its modest contribution of T3. The defect is unmasked when a hypothyroid patient is treated with levothyroxine and deiodination of T4 must produce not 80% but 100% of the daily T3. When hypothyroid, they respond better to T3 + T4 treatment than to T4 alone.
Resistance to Thyroid Hormone, Part 2
Those physicians who treat patients with normal TSH and normal freeT4 based on clinical grounds… could they have valid reasons for doing so? Is there a situation in which a person could be physiologically hypothyroid despite having plenty of T4 and normal TSH? Well, sure – we’ve just reviewed a variety of cases and conditions.
Tabor’s defines “hypothyroidism” as the clinical consequences of inadequate thyroid hormone in the body”1; perhaps “active-hormone” would be more precise. The deiodination of thyroid hormones is purposefully directed, and it is the primary means of regulating the biological activity of thyroid hormone.20 As cited above and repeatedly supported, altered deiodination causes symptomatic “disruption of thyroid hormone signaling,” even when T3 levels remain within the normal range.171
This physiological hypothyroidism occurs in the tissues, at the second level of regulation, not at the HP-T axis. Its laboratory hallmark is a low tT3/RT3 ratio—neither high TSH nor low freeT4… not even low T3. Indeed, even with normal freeT4 and TSH, the developmental defects in hereditary thyroid hormone metabolism defect267 and the high death rate in ESS/NTI prove dysfunctional deiodination can be very serious.
Many causes of acquired dysfunction of deiodination are listed above, from emotional stress to metabolic syndrome, drugs, and toxins. If the resulting thyroid hormone derangement is clinically relevant, by what name can we call this condition? The term “type 2-hypothyroidism” has been proposed275: It seems appropriate, as adult-onset diabetes with lots of insulin that “doesn’t work” is called type 2-diabetes.
If this were a valid clinical problem, our first indicators would be patients who fail treatment with T4-only, which indeed happens.134,135,136,137 If the problem occurred in people who were not hypothyroid, the concept could be lifted out of the Critical Care Unit and applied to ambulatory patients—and it has been.207,276
If dysfunctional deiodination causes clinical symptoms, as proponents of type-2 hypothyroidism assert, we should expect to see low tT3/RT3 demonstrated in studies of patients with otherwise unexplained symptoms—such as chronic fatigue. This also has been reported.276
More evidence may be found in studies of euthyroid Hashimoto’s disease (AIT), which is strongly associated with excessive RT3 (p<0.00002)147—likely due to (intra-thyroidal) cytokines.277,278,279,280,281 Although AIT is often judged inconsequential except as a risk for future hypothyroidism, patients have increased miscarriages248 and symptoms with decreased quality-of-life that correlate with anti-TPO antibody level, not TSH.282 Conversely, chronically fatigued patients are significantly more likely to have AIT.283
If dysfunctional deiodination causes over-production of RT3 and low tT3/RT3, then we expect that T3-replacement, which as Refetoff says “bypasses the defect,” should correct these patients’ imbalance and restore a desirable ratio. This also has been demonstrated.219
If correcting the tT3/RT3 ratio relieves patients’ symptoms, we should see reports claiming T3 treatment helps such symptomatic “euthyroid” people. These too we have, in a wide variety of settings. The publications range from animal research on demyelinating diseases284,285 to ADHD in hereditary RTH286; from depression287,288,289,290 and dementia-related behavior problems219 to heart failure.291,292 They also include toxin-induced euthyroid sick syndrome (NTI)293 and simply “low metabolism.”294,295
The limitations of this review prevent taking the discussion of type 2-hypothyroidism beyond this point. The prescription of T3 for euthyroid patients is currently discouraged by authorities,37 despite its apparent success: After average follow-up of 6.9 years, 58% of “inappropriate users” of desiccated thyroid were still taking it.111 It is my experience that T3 treatment for “type-2 hypothyroidism” is highly rewarding for patients, though it can be risky for a physician whose state board of medicine begins an inquiry.
In closing, I’d like to remind you of the four most important points I have tried to prove in this review:
1. Thyroid hormone doses should be divided at least every 12 hours.
2. Therapeutic blood levels must be tested according to peak/trough fluctuations; preferably at mid-dose.
3. The ratio of tT3/ RT3 is the most accurate measure of the actual thyroid hormone function in the body.
4. Some people need to take T3 along with T4 for their best clinical results.
It is possible to achieve truly remarkable results with the simple suggestions I’ve offered. I would love working with the thyroid even if I did not personally have Hashimoto’s. I hope you will find this work rewarding without being motivated by needing it yourself!




