By Thierry Hertoghe, MD
Editor’s Note: In Part 1 of this article (Townsend Letter, April 2020), Dr. Hertoghe discussed diagnosis, associated pathologies, and underlying causes of Hashimoto’s thyroiditis. He also presented details about dietary measures that can reduce antithyroid antibody levels. Part 2 continues with more treatments for underlying causes.
Causes to Treat: Nutritional Deficiencies
Nutritional therapies to treat nutritional deficiencies may decrease thyroid antibody levels by 20-50%.
Selenium and vitamin D supplementations: Patients with Hashimoto’s thyroiditis have been reported to have significantly lower selenium 315-316 and vitamin D 317-339 levels than controls without the disorder. On the other hand, daily selenium supplementation can decrease the levels of thyroid antibodies by 20 to 30%, 8,340-344 particularly if the level is low-normal or below the normal limit. The same is valid for vitamin D supplements.345-354
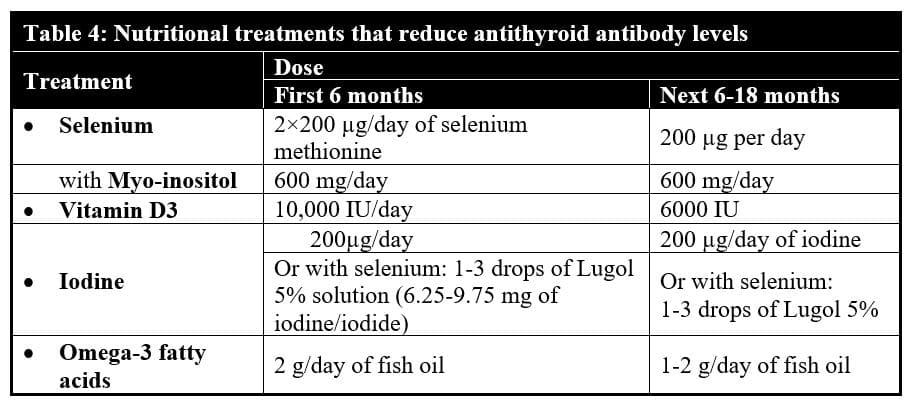
What are adequate doses? In adults, the dosage of selenium supplements should be at least 200 µg a day for several months and higher in the case of a noted deficiency in this trace element. Concomitant intake of 600 mg a day of myo-inositol has shown to increase the efficacy of selenium supplements to further reduce thyroid antibody levels. 8,343-344 Efficient doses of vitamin D3 supplements are 4000 IU a day at minimum, but higher doses between 10,000 IU (lean persons) to 20,000 IU a day (obese persons and intensive sports practitioners) may be more effective.
Iodine supplementation may be crucial, as about one-quarter of studies have shown that low iodine levels, especially within the thyroid gland,355-356 have been associated with higher levels of antithyroid autoimmune antibodies.355-360 At least one study has shown iodine supplementation to reduce the levels of anti-thyroid antibodies.361 However, the majority of epidemiological studies have reported that high iodine intake increases the risk of autoimmune thyroiditis in iodine-deficient areas.362-370 For this reason, if iodine is given alone, it may be better to limit the dose to 200 µg a day, as a study showed that in thyroxine-treated patients the production of thyroid antibodies was not induced at that dose.371 If higher doses of iodine are requested, it should be administered along with selenium. Selenium appears to reduce markedly the risk of inducing autoimmune thyroiditis and other toxic effects on the thyroid with excessive iodine intake.372-375
Omega-3 polyunsaturated fatty acid supplements: Adding 2 g per day of fish oil for patients who don’t regularly eat fatty fishes might help reduce autoimmune antibodies, just as fatty food consumption does. Daily omega-3 supplementation has been shown to prevent and reduce the autoimmune antibody production in mice with autoimmune lupus376-377 and glomerulonephritis.380 In vitro studies of dendritic cells show that adding docosahexaenoic acid, one of the major omega-3 fatty acids of fish oil, prevents development of experimental autoimmune encephalomyelitis (multiple sclerosis), another autoimmune disorder.379
Thus, after confirmation that nutritional levels are low, low-normal, or even average in laboratory tests (but not if these levels are high-normal or above the upper limit), I suggest the intake of higher amounts of the above-mentioned nutrients the first four to six months.
Table 4 gives an overview of the dosages of the nutritional treatments that can efficiently calm down the autoimmune thyroiditis.
Cause to Treat: Hormone Deficiencies
Hormone treatments can decrease thyroid antibody levels by 20-70%.
Thyroid treatment: Thyroid therapy is necessary not only to reduce thyroid antibodies380-394 but to relieve the patient’s hypothyroid symptoms and the risks and severity of psychological and somatic disorders that often accompany autoimmune thyroiditis. Many studies have shown the efficacy of thyroxine treatment. The best efficacy is reached when the dose is high enough to suppress the TSH level in the serum.
What is the best thyroid therapy? In most cases, I suggest desiccated thyroid extracts such as Armour, Erfa, or Nature Thyroid (doses between 30-180 mg/day) because they work better, in my experience, due to their high content in T3, T2, T1, and T0, which are not found in treatments with thyroxine alone. Be careful with patients who are allergic to pork. Most desiccated thyroid are of porcine origin and should be avoided by patients with pork meat allergy. In this case, synthetic T3-T4 combinations might be an acceptable alternative, but are not as efficient as desiccated thyroid. Triiodothyronine alone is not indicated because of the lack of persistence of beneficial effects over a 24-hour period (except if administered in 5 divided doses per day). Thyroxine alone may help on the condition that it clearly reduces the TSH level, even suppresses it. Titrate the dose up to just below the level that causes signs and symptoms of thyroid excess.
Glucocorticoid and DHEA treatments: One of the roles of cortisol is to prevent autoantibody production. In cases of cortisol deficiencies, such as Addison’s disease, the risk of autoimmune thyroiditis considerably increases. 126 This explains why glucocorticoid treatments may significantly decrease the production of antithyroid antibodies but, in my experience, rarely eradicates it totally at physiological doses. Hydrocortisone (bioidentical cortisol) at doses of 15-35 mg/day in at least two divided doses (more in the morning, less at lunch) is recommended for patients with adrenal deficiency and autoimmune thyroiditis. When levels of antithyroid antibodies are high, treatment with a long-acting synthetic derivative of cortisol during the first six months may be more efficient to reduce the antithyroid antibody titers.97,393-395 Prednisolone, which has more persistent effects over the next 24 hours than bioidentical hydrocortisone whose biological action fades after 6-9 hours, is then a good choice.
Add anabolic DHEA (dehydroepiandrosterone) to any glucocorticoid treatment in doses that are at least equivalent in milligrams to the dose of hydrocortisone that is given (15-35 mg/day). The anabolic actions of DHEA neutralize any excessive catabolism from the glucocorticoid treatment and have the additional benefit of further reducing antithyroid antibodies, as shown in women with Hashimoto’s thyroiditis and premature ovarian failure. In these women, DHEA sulfate levels were found to be significantly lower than in women without Hashimoto’s and normalized with a substantial reduction of anti-thyroperoxidase antibodies at 30 mg/day of DHEA. DHEA treatment also normalizes the natural killer cell toxicity, which is deficient, in patients with Hashimoto’s thyroiditis.397
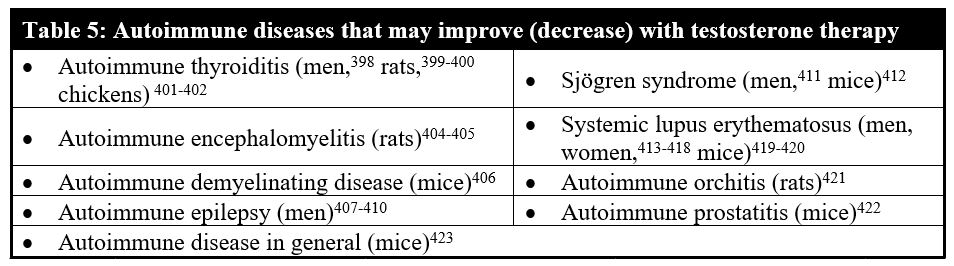
Testosterone therapy, along with female hormone treatments in women, has been reported to oppose the development of various types of autoimmune disease. In autoimmune thyroiditis, a significantly lower testosterone to estradiol ratio is found in men.148 Testosterone therapy, on the other hand, has been shown to reduce antithyroid peroxidase and antithyroglobulin antibodies in men and animals with the disorder.398-402 In women with autoimmune lupus, the testosterone is also significantly lower in the serum.403
Table 5 gives an overview of various autoimmune pathologies in which testosterone therapy was shown to reduce the autoimmune antibody levels.
As discussed above, the reason autoimmune thyroiditis is 5 to 10 times more common in women than men may be due to their much lower testosterone levels, which leave them less protected against autoimmune dysregulation. Regarding lupus, another autoimmune disorder, testosterone levels are lower in women who have lupus than in women without it, and testosterone therapy has been reported to reduce the production of autoimmune antibodies in women with lupus. I have not (yet) found studies on testosterone therapy for autoimmune thyroiditis in women.
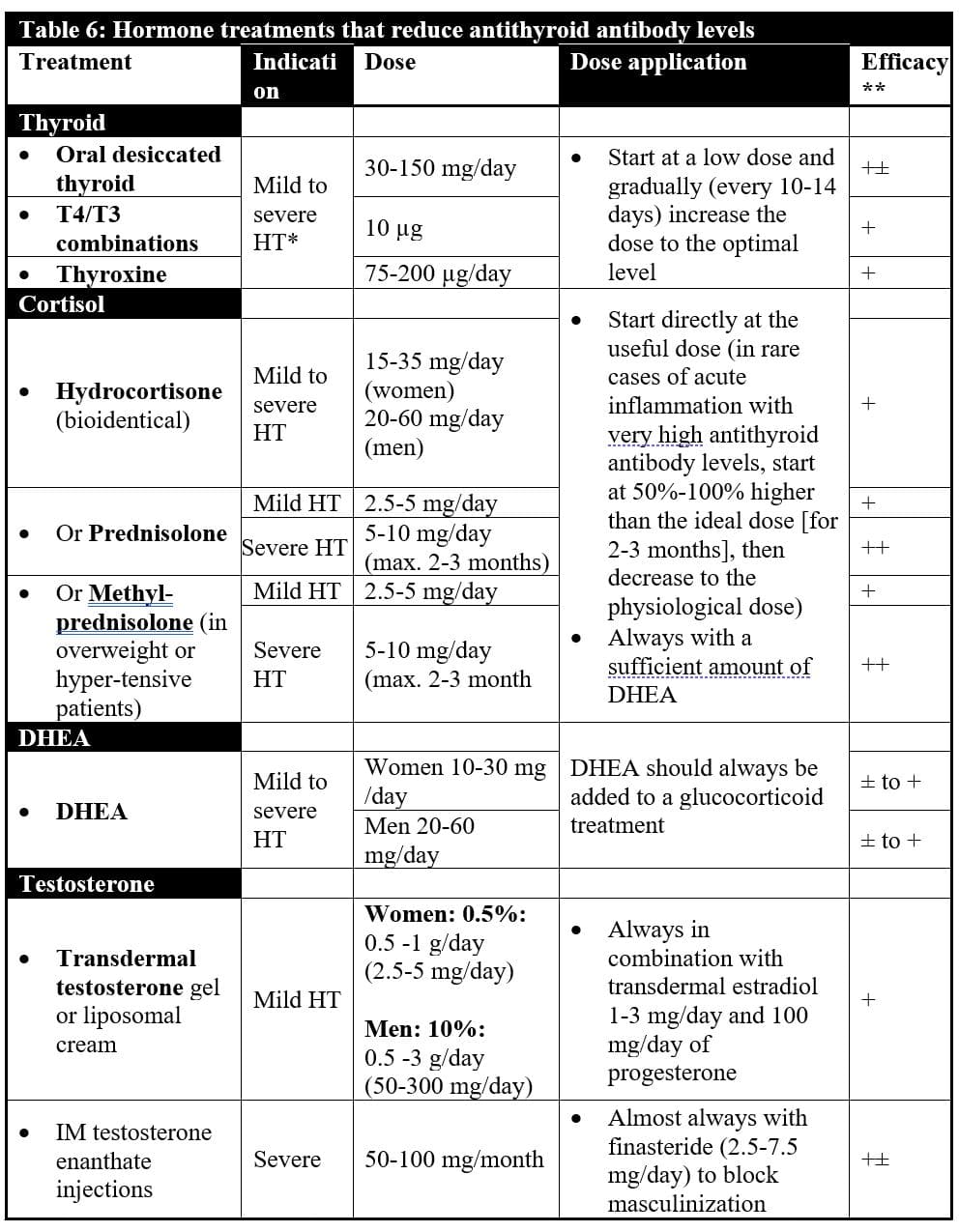
However, if testosterone therapy is administered to a female patient, it should always be done in combination with sufficient female hormone therapies to avoid masculinization. Estrogen and progesterone therapy protect women against hair loss, body hair overgrowth, acne, and other undesirable effects of testosterone therapy administered alone. In case high amounts of intramuscular testosterone injections are administered, finasteride, a progesterone derivative, which reduces the conversion of testosterone to the masculinizing dihydrotestosterone, may have to be added and is safe only in presence of testosterone supplementation.
Table 6 overviews the most important hormone treatments for Hashimoto’s thyroiditis.
Note: *HT = Hashimoto’s thyroiditis: mild: antithyroid antibody levels reach a maximum of 10 times the upper limit; severe: antithyroid antibody levels are far higher than 10 times the upper limit; ** efficacy to reduce autoimmune antibodies.
Causes to Treat: Viral, Bacterial, and Yeast Infections
Infections by microorganisms have been reported to cause or contribute to the development of autoimmune thyroiditis. Both a leaky gut, which permits these microorganisms to penetrate into the body, and an inefficient immune system contribute to make a patient’s thyroid gland prone to infection by viruses, bacteria, and yeast, which trigger autoimmune reactions. In autoimmune thyroiditis, two immune failures have been discovered: a defect in the number of CD8(+) suppressor lymphocytes424 and a decrease in efficacy (toxicity) of natural killer cells.425-426 CD8(+) suppressor lymphocytes are those that oppose the development of autoimmune thyroiditis. Natural killer cells serve to contain viral infections to provide time for the immune system to produce antigen-specific cytotoxic T cells that respond to the invaders and clear the infection. Natural killer cells control viral infections by secreting interferon γ and tumor necrosis factor α.
Viruses seem to trigger or amplify autoimmune thyroiditis more than other infectious agents.427-429 Especially herpes viruses, particularly the Epstein-Barr virus430-435 (also called herpes virus 4) that causes mononucleosis seems to be worst, but the Herpes virus436-437 that causes herpes labialis has also been incriminated as facilitating Hashimoto’s thyroiditis. The Hepatitis C virus,438 parvovirus,439 human T-cell lymphotropic virus type 1,440-442 and HIV virus443-444 have also been associated with autoimmune thyroiditis.
Yeast is another trigger of autoimmune diseases, and, therefore, potentially also of autoimmune thyroiditis. Patients with autoimmune diabetes, for example, are four times more likely to have anti-Candida (enolase) IgG antibodies,445 and twice more likely to have Candida albicans overgrowth in the stools.446 Furthermore, experimental autoimmune encephalomyelitis is considerably aggravated if mice are beforehand infected by Candida. Bacteria that have been associated with thyroiditis include streptococci, staphylococci,437 Yersinia, Borrelia (Lyme disease),448-449 and Helicobacter pylori.435 Among the parasites that have been associated with autoimmune thyroiditis is Treponema gondii.450

How to avoid getting the thyroid invaded by microorganisms that trigger autoimmune thyroiditis? First of all, by blocking the passage of these microorganisms through a leaky gut by a healthy diet, as explained earlier in this article. Second, by restoring an optimal immune system by hormone and nutritional supplementations (of any deficiency) so that even if microorganisms pass through the gut wall, they are destroyed by the body’s natural defenses. Thyroid therapy is a strong immune booster that has been shown to increase natural killer cell toxicity451-453 and increase both the number of CD4 helper and CD8 suppressor cells.454-455
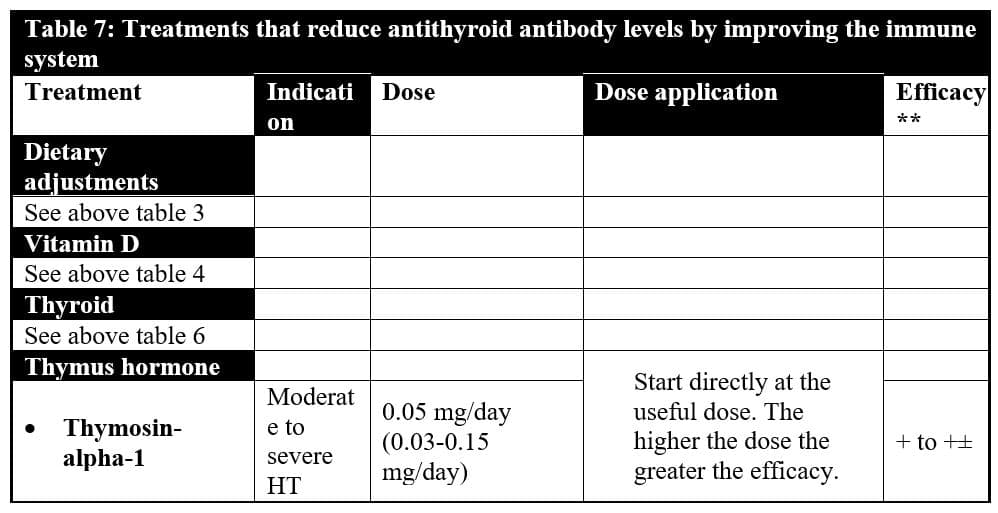
Moreover, daily subcutaneous injections of thymosin-alpha-1, probably the body’s most potent immune-enhancing hormone may be an additional powerful tool. Research has demonstrated that thymosin-alpha-1 strongly opposes the development of autoimmune thyroiditis in mice prone to produce antithyroid antibodies. However, in mice, relatively resistant to autoimmunity, thymosin-alpha-1 may trigger a mild form of autoimmune thyroiditis.456-457 For this reason, treatment with thymosin-alpha-1 is probably and mainly indicated in patients with high titers of thyroid antibodies.
Thymosin-alpha-1 activates the natural killer cell activity.458-460 In vitro, it has been shown to inhibit the proliferation of the HIV virus in infection of macrophages and polymorphonuclear cells by activating CD8(+) cells so powerfully that some researchers think it makes a re-evaluation of the approach to antiretroviral therapy necessary.461 In Lyme disease, this treatment is, in my experience, efficient in reducing the aggressiveness of the disease, much more than prolonged use of antibiotics that has toxic effects that thymosin-alpha-1 does not have. I cannot stress enough that the best therapies are those that treat the cause (the immune deficiency due to a lack of thymus hormones, for example) than the consequences (providing long-term antibiotics to kill intracellular microorganisms which install themselves when there is an immune deficiency). Furthermore, thymosin-alpha-1 is anti-inflammatory, a beneficial effect in autoimmune diseases.
Causes to Treat: Pollutants
Breathe pure air. Pollutants trigger the development of autoimmune thyroiditis by attacking the immune system and tissues. There are so many toxins that we cannot list them all, but let’s cover some of them.
Smoking increases the risk of autoimmune thyroiditis.462-463 High cadmium levels have been associated with autoimmune thyroiditis,464 as has radioactive fallout from Chernobyl, for example.465-466

to give to patients to lessen the toxic load.
Endocrine disruptors such as polychlorinated biphenyls (PCBs), used as coolants and insulating fluids (transformer oil) for transformers and capacitors or plasticizers in cement, are so persistent that even if a baby receives them through breast-feeding from his mother, they remain in the body as a young adult. Young adults who were breastfed as babies have been reported to have higher levels of the various types of persistent PCBs and antithyroid peroxidase antibodies than individuals who were not breastfed.467
Conclusion
Because of its frequency and adverse impact, Hashimoto’s thyroiditis should be systematically screened and treated in women. The treatment of autoimmune thyroiditis (particularly Hashimoto’s thyroiditis) is generally a combination of therapies consisting of, at least, dietary adjustments, nutritional therapies (selenium and vitamin D3 being the most important ones), and reduction of any toxic overload. However, in more severe or treatment-resistant forms with highly elevated antithyroid antibody levels, hormone therapies, such as thyroid, glucocorticoid, DHEA, and/or testosterone (associated with female hormone therapy in women), are generally inevitable, and optimization of the immune system might be necessary. In case the patient is hypothyroid, thyroid therapy becomes essential to treat the hypothyroidism and can considerably reduce antithyroid antibody levels too.
Click here for COMPLETE REFERENCES (Part 1, Part 2)

Born in Antwerp, Belgium, Dr. Hertoghe practices his medicine in his clinic in Brussels. With his sister, Dr. Thérèse Hertoghe, they proudly represent the fourth successive generation of physicians working with hormonal treatments – and this since 1892 (after Eugène Hertoghe former vice president of the Royal Academy of Medicine in Belgium, and Luc and Jacques Hertoghe, endocrinologists). Dr. Thierry Hertoghe devotes his life to the promotion of a better, patient oriented, and evidence-based medicine.
Author of numerous books, Dr. Thierry Hertoghe also travels a lot to take part in numerous conferences and congresses throughout the world. He co-organizes many of these specialized gatherings and holds important positions in several international and national medical organizations (which usually tend to fight against aging). He is the president of the International Hormone Society (over 2500 physicians), and of the World Society of Anti-Aging Medicine (over 7000 physicians), as well as the supervisor of two important postacademic trainings for doctors.
http://www.hertoghe.eu
References for Part Two
- Rasic-Milutinovic Z, Jovanovic D, Bogdanovic G, Trifunovic J, Mutic J. Potential Influence of Selenium, Copper, Zinc and Cadmium on L-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp Clin Endocrinol Diabetes. 2017 Feb;125(2):79-85.
- Erdal M, Sahin M, Hasimi A, Uckaya G, Kutlu M, Saglam K. Trace element levels in Hashimoto thyroiditis patients with subclinical hypothyroidism. Biol Trace Elem Res. 2008 Summer;123(1-3):1-7.
- Krysiak R, Szkróbka W, Okopień B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp Clin Endocrinol Diabetes. 2019 Jul;127(7):417-422.
- Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto’s thyroiditis: A pilot study. Pharmacol Rep. 2019 Apr;71(2):243-247.
- De Pergola G, Triggiani V, Bartolomeo N, Giagulli VA, Anelli M, Masiello M, Candita V, De Bellis D, Silvestris F. Low 25 hydroxyvitamin D levels are independently associated with autoimmune thyroiditis in a cohort of apparently healthy overweight and obese subjects. Endocr Metab Immune Disord Drug Targets. 2018;18(6):646-652.
- Bakr HG, Meawed TE. Relevance of 25 (OH) Vitamin D deficiency on Hashimoto’s Thyroiditis. Egypt J Immunol. 2017 Jun;24(2):53-62.
- Krysiak R, Szkróbka W, Okopień B. Moderate-dose simvastatin therapy potentiates the effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and vitamin D insufficiency. Pharmacol Rep. 2018 Feb;70(1):93-97.
- Kim M, Song E, Oh HS, Park S, Kwon H, Jeon MJ, Kim WG, Kim WB, Shong YK, Kim TY. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine. 2017 Nov;58(2):332-339.
- Ucan B, Sahin M, Sayki Arslan M, Colak Bozkurt N, Kizilgul M, Güngünes A, Cakal E, Ozbek M. Vitamin D treatment in patients with Hashimoto’s thyroiditis may decrease the development of hypothyroidism. Int J Vitam Nutr Res. 2016 Feb;86(1-2):9-17.
- Nalbant A, Aydin A, Karacan A, Onmez A, Tamer A, Cinemre H. Association of vitamin D insufficiency/deficiency with thyroid artery Doppler ultrasonography in patients with Hashimoto thyroiditis. Pak J Med Sci. 2017 Mar-Apr;33(2):295-299.
- Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. 2017 Apr;125(4):229-233. (titers of thyroid antibodies inversely correlated with 25-hydroxyvitamin D levels
- Kim D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones (Athens). 2016 Jul;15(3):385-393.
- Şıklar Z, Karataş D, Doğu F, Hacıhamdioğlu B, İkincioğulları A, Berberoğlu M. Regulatory T Cells and Vitamin D Status in Children with Chronic Autoimmune Thyroiditis. J Clin Res Pediatr Endocrinol. 2016 Sep 1;8(3):276-81.
- Giovinazzo S, Vicchio TM, Certo R, Alibrandi A, Palmieri O, Campennì A, Cannavò S, Trimarchi F, Ruggeri RM. Vitamin D receptor gene polymorphisms/haplotypes and serum 25(OH)D(3) levels in Hashimoto’s thyroiditis. Endocrine. 2017 Feb;55(2):599-606.
- Metwalley KA, Farghaly HS, Sherief T, Hussein A. Vitamin D status in children and adolescents with autoimmune thyroiditis. J Endocrinol Invest. 2016 Jul;39(7):793-7.
- Maciejewski A, Wójcicka M, Roszak M, Losy J, Łącka K. Assessment of vitamin D level in autoimmune thyroiditis patients and a control group in the Polish population. Adv Clin Exp Med. 2015 Sep-Oct;24(5):801-6.
- Krysiak R, Kowalcze K, Okopien B. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur J Clin Nutr. 2016 May;70(5):637-9.
- Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, Tzortzinis AA. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell J Nucl Med. 2015 Sep-Dec;18(3):222-7.
- Evliyaoğlu O, Acar M, Özcabı B, Erginöz E, Bucak F, Ercan O, Kucur M. Vitamin D deficiency and Hashimoto’s thyroiditis in children and adolescents: a critical vitamin D level for this association? J Clin Res Pediatr Endocrinol. 2015 Jun;7(2):128-33.
- Arslan MS, Topaloglu O, Ucan B, Karakose M, Karbek B, Tutal E, Caliskan M, Ginis Z, Cakal E, Sahin M, Ozbek M, Delibasi T. Isolated vitamin D deficiency is not associated with nonthyroidal illness syndrome, but with thyroid autoimmunity. ScientificWorldJournal. 2015;2015:239815.
- Shin DY, Kim KJ, Kim D, Hwang S, Lee EJ. Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med J. 2014 Mar;55(2):476-81.
- Unal AD, Tarcin O, Parildar H, Cigerli O, Eroglu H, Demirag NG. Vitamin D deficiency is related to thyroid antibodies in autoimmune thyroiditis. Cent Eur J Immunol. 2014;39(4):493-7.
- Bozkurt NC, Karbek B, Ucan B, Sahin M, Cakal E, Ozbek M, Delibasi T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr Pract. 2013 May-Jun;19(3):479-84.
- Camurdan OM, Döğer E, Bideci A, Celik N, Cinaz P. Vitamin D status in children with Hashimoto thyroiditis. J Pediatr Endocrinol Metab. 2012;25(5-6):467-70.
- Tamer G, Arik S, Tamer I, Coksert D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid. 2011 Aug;21(8):891-6.
- de Farias CR, Cardoso BR, de Oliveira GM, de Mello Guazzelli IC, Catarino RM, Chammas MC, Cozzolino SM, Knobel M. A randomized-controlled, double-blind study of the impact of selenium supplementation on thyroid autoimmunity and inflammation with focus on the GPx1 genotypes. J Endocrinol Invest. 2015 Oct;38(10):1065-74.
- Zhu L, Bai X, Teng WP, Shan ZY, Wang WW, Fan CL, Wang H, Zhang HM. [Effects of selenium supplementation on antibodies of autoimmune thyroiditis]. Zhonghua Yi Xue Za Zhi. 2012 Aug 28;92(32):2256-60.
- Bhuyan AK, Sarma D, Saikia UK. Selenium and the thyroid: A close-knit connection. Indian J Endocrinol Metab. 2012 Dec;16(Suppl 2):S354-5.
- Ferrari SM, Fallahi P, Di Bari F, Vita R, Benvenga S, Antonelli A. Myo-inositol and selenium reduce the risk of developing overt hypothyroidism in patients with autoimmune thyroiditis. Eur Rev Med Pharmacol Sci. 2017 Jun;21(2 Suppl):36-42.
- Nordio M, Basciani S. Treatment with myo-inositol and selenium ensures euthyroidism in patients with autoimmune thyroiditis. Int J Endocrinol. 2017;2017:2549491.
- Chahardoli R, Saboor-Yaraghi AA, Amouzegar A, Khalili D, Vakili AZ, Azizi F. Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto’s thyroiditis? A double blind, randomized clinical trial. Horm Metab Res. 2019 May;51(5):296-301.
- Krysiak R, Kowalcze K, Okopień B. Selenomethionine potentiates the impact of vitamin D on thyroid autoimmunity in euthyroid women with Hashimoto’s thyroiditis and low vitamin D status. Pharmacol Rep. 2019 Apr;71(2):367-373.
- Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto’s thyroiditis: A pilot study. Pharmacol Rep. 2019 Apr;71(2):243-247.
- Krysiak R, Szkróbka W, Okopień B. Moderate-dose simvastatin therapy potentiates the effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and vitamin D insufficiency. Pharmacol Rep. 2018 Feb;70(1):93-97.
- Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. 2017 Apr;125(4):229-233.
- Simsek Y, Cakır I, Yetmis M, Dizdar OS, Baspinar O, Gokay F. Effects of Vitamin D treatment on thyroid autoimmunity. J Res Med Sci. 2016 Oct 18;21:85.
- Krysiak R, Kowalcze K, Okopien B. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur J Clin Nutr. 2016 May;70(5):637-9.
- Krysiak R, Kowalcze K, Okopień B. The effect of vitamin D on thyroid autoimmunity in euthyroid men with autoimmune thyroiditis and testosterone deficiency. Pharmacol Rep. 2019 Oct;71(5):798-803.
- Ucan B, Sahin M, Sayki Arslan M, Colak Bozkurt N, Kizilgul M, Güngünes A, Cakal E, Ozbek M. Vitamin D treatment in patients with Hashimoto’s thyroiditis may decrease the development of hypothyroidism. Int J Vitam Nutr Res. 2016 Feb;86(1-2):9-17.
- Mazokopakis EE, Papadomanolaki MG, Tsekouras KC, Evangelopoulos AD, Kotsiris DA, Tzortzinis AA. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell J Nucl Med. 2015 Sep-Dec;18(3):222-7.
- Kim M, Song E, Oh HS, Park S, Kwon H, Jeon MJ, Kim WG, Kim WB, Shong YK, Kim TY. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine. 2017 Nov;58(2):332-339.
- Andersen S, Iversen F, Terpling S, Pedersen KM, Gustenhoff P, Laurberg P. Iodine deficiency influences thyroid autoimmunity in old age–a comparative population-based study. Maturitas. 2012 Jan;71(1):39-43.
- Ergür AT, Evliyaoğlu O, Şıklar Z, Bilir P, Öcal G, Berberoğlu M. Evaluation of thyroid functions with respect to iodine status and TRH test in chronic autoimmune thyroiditis. J Clin Res Pediatr Endocrinol. 2011;3(1):18-21.
- Bayram F, Beyazyildiz A, Gökçe C, Budak N, Erdoğan N, Kurtoğlu S, Kula M, Unlühizarci K, Keleştimur F. The prevalence of iodine deficiency, serum thyroglobulin, anti-thyroglobulin and thyroid peroxidase antibody levels in the urban areas of Kayseri, Central Anatolia. Exp Clin Endocrinol Diabetes. 2009 Feb;117(2):64-8.
- Jonckheer MH, Vanhaelst L, Deconinck F, Michotte Y. Atrophic autoimmune thyroiditis: relationship between the clinical state and intrathyroidal iodine as measured in vivo in man. J Clin Endocrinol Metab. 1981 Sep;53(3):476-9.
- Yamamoto M, Saito S, Sakurada T, Kaise K, Kaise N, Yoshida K, Kitaoka H, Fukazawa H, Yoshinaga K. Abnormalities of iodine and protein in the thyroid of patients with Hashimoto’s thyroiditis. Tohoku J Exp Med. 1981 Nov;135(3):323-30.
- Mazziotti G, Premawardhana LD, Parkes AB, Adams H, Smyth PP, Smith DF, Kaluarachi WN, Wijeyaratne CN, Jayasinghe A, de Silva DG, Lazarus JH. Evolution of thyroid autoimmunity during iodine prophylaxis–the Sri Lankan experience. Eur J Endocrinol. 2003 Aug;149(2):103-10.
- Flores-Rebollar A, Moreno-Castañeda L, Vega-Servín NS, López-Carrasco G, Ruiz-Juvera A. Prevalence of autoimmune thyroiditis and thyroid dysfunction in healthy adult Mexicans with a slightly excessive iodine intake. Nutr Hosp. 2015 Aug 1;32(2):918-24.
- Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011 Jun;164(6):943-50.
- Bastemir M, Emral R, Erdogan G, Gullu S. High prevalence of thyroid dysfunction and autoimmune thyroiditis in adolescents after elimination of iodine deficiency in the Eastern Black Sea Region of Turkey. Thyroid. 2006 Dec;16(12):1265-71.
- Zois C, Stavrou I, Kalogera C, Svarna E, Dimoliatis I, Seferiadis K, Tsatsoulis A. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid. 2003 May;13(5):485-9.
- Papanastasiou L, Alevizaki M, Piperingos G, Mantzos E, Tseleni-Balafouta S, Koutras DA. The effect of iodine administration on the development of thyroid autoimmunity in patients with nontoxic goiter. Thyroid. 2000 Jun;10(6):493-7.
- Reinhardt W, Luster M, Rudorff KH, Heckmann C, Petrasch S, Lederbogen S, Haase R, Saller B, Reiners C, Reinwein D, Mann K. Effect of small doses of iodine on thyroid function in patients with Hashimoto’s thyroiditis residing in an area of mild iodine deficiency. Eur J Endocrinol. 1998 Jul;139(1):23-8.
- Boyages SC, Bloot AM, Maberly GF, Eastman CJ, Li M, Qian QD, Liu DR, van der Gaag RD, Drexhage HA. Thyroid autoimmunity in endemic goitre caused by excessive iodine intake. Clin Endocrinol (Oxf). 1989 Oct;31(4):453-65.
- Fragu P, Schlumberger M, Tubiana M. Thyroid iodine content and serum thyroid hormone levels in autoimmune thyroiditis: effect of iodide supplementation. J Nucl Med. 1985 Feb;26(2):133-9.
- Meng W, Schindler A, Spieker K, Krabbe S, Behnke N, Schulze W, Blümel C. Iodine therapy for iodine deficiency goiter and autoimmune thyroiditis. A prospective study. Med Klin (Munich). 1999 Nov 15;94(11):597-602.
- Rink T, Schroth HJ, Holle LH, Garth H. Effect of iodine and thyroid hormones in the induction and therapy of Hashimoto’s thyroiditis. Nuklearmedizin. 1999;38(5):144-9.
- Xue H, Wang W, Li Y, Shan Z, Li Y, Teng X, Gao Y, Fan C, Teng W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr J. 2010;57(7):595-601.
- Selenium treatment reduces in mice Xu J, Liu XL, Yang XF, Guo HL, Zhao LN, Sun XF. Supplemental selenium alleviates the toxic effects of excessive iodine on thyroid. Biol Trace Elem Res. 2011 Jun;141(1-3):110-8.
- Xu J, Yang XF, Guo HL, Hou XH, Liu LG, Sun XF. Selenium supplement alleviated the toxic effects of excessive iodine in mice. Biol Trace Elem Res. 2006 Summer;111(1-3):229-38.
- Beckett GJ, Nicol F, Rae PW, Beech S, Guo Y, Arthur JR. Effects of combined iodine and selenium deficiency on thyroid hormone metabolism in rats. Am J Clin Nutr. 1993 Feb;57(2 Suppl):240S-243S.
- Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol. 2010 May 1;184(9):5280-6.
- Robinson DR, Xu LL, Tateno S, Guo M, Colvin RB. Suppression of autoimmune disease by dietary n-3 fatty acids. J Lipid Res. 1993 Aug;34(8):1435-44.
- 5: Pestka JJ. n-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot Essent Fatty Acids. 2010 Apr-Jun;82(4-6):251-8.
- Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011 Jul;25(5):872-82.
- Korzeniowska K, Jarosz-Chobot P, Szypowska A, Ramotowska A, Fendler W, Kalina-Faska B, Szadkowska A, Mlynarski W, Mysliwiec M. L-thyroxine stabilizes autoimmune inflammatory process in euthyroid nongoitrous children with Hashimoto’s thyroiditis and type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2013;5(4):240-4.
- Yamauchi K, Yamada T, Sato A, Inazawa K, Aizawa T. Elevation of serum immunoglobulin G in Hashimoto’s thyroiditis and decrease after treatment with L-thyroxine in hypothyroid patients. Intern Med. 2010;49(4):267-71.
- Guclu F, Ozmen B, Kirmaz C, Kafesciler SO, Degirmenci PB, Taneli F, Hekimsoy Z. Down-regulation of the auto-aggressive processes in patients with hypothyroid Hashimoto’s thyroiditis following substitutive treatment with L-thyroxine. Eur Cytokine Netw. 2009 Mar;20(1):27-32.
- Revelli A, Casano S, Piane LD, Grassi G, Gennarelli G, Guidetti D, Massobrio M. A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: effects of levothyroxine, acetyl-salicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol. 2009 Nov 27;7:137.
- Schmidt M, Voell M, Rahlff I, Dietlein M, Kobe C, Faust M, Schicha H. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008 Jul;18(7):755-60.
- Aksoy DY, Kerimoglu U, Okur H, Canpinar H, Karaağaoğlu E, Yetgin S, Kansu E, Gedik O. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005 Jun;52(3):337-43.
- Khoo DH, Eng PH, Ho SC, Fok AC. Differences in the levels of TSH-binding inhibitor immunoglobulins in goitrous and agoitrous autoimmune thyroiditis after twelve months of L-thyroxine therapy. Clin Endocrinol (Oxf). 1999 Jul;51(1):73-9.
- Romaldini JH, Biancalana MM, Figueiredo DI, Farah CS, Mathias PC. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996 Jun;6(3):183-8.
- Rieu M, Richard A, Rosilio M, Laplanche S, Ropion V, Fombeur JP, Berrod JL. Effects of thyroid status on thyroid autoimmunity expression in euthyroid and hypothyroid patients with Hashimoto’s thyroiditis. Clin Endocrinol (Oxf). 1994 Apr;40(4):529-35
- Chiovato L, Marcocci C, Mariotti S, Mori A, Pinchera A. L-thyroxine therapy induces a fall of thyroid microsomal and thyroglobulin antibodies in idiopathic myxedema and in hypothyroid, but not in euthyroid Hashimoto’s thyroiditis. J Endocrinol Invest. 1986 Aug;9(4):299-305.
- Padberg S, Heller K, Usadel KH, Schumm-Draeger PM. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001 Mar;11(3):249-55.
- Rink T, Schroth HJ, Holle LH, Garth H. Effect of iodine and thyroid hormones in the induction and therapy of Hashimoto’s thyroiditis] Nuklearmedizin. 1999;38(5):144-9.
- Chiovato L, Marcocci C, Mariotti S, Mori A, Pinchera A. L-thyroxine therapy induces a fall of thyroid microsomal and thyroglobulin antibodies in idiopathic myxedema and in hypothyroid, but not in euthyroid Hashimoto’s thyroiditis. J Endocrinol Invest. 1986 Aug;9(4):299-305.
- Revelli A, Casano S, Piane LD, Grassi G, Gennarelli G, Guidetti D, Massobrio M. A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: effects of levothyroxine, acetyl-salicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol. 2009 Nov 27;7:137.
- Ito S, Tamura T, Nishikawa M. Effects of desiccated thyroid, prednisolone and chloroquine on goiter and antibody titer in chronic thyroiditis. Metabolism. 1968 Apr;17(4):317-25.
- Litwicka K, Arrivi C, Varricchio MT, Mencacci C, Greco E. In women with thyroid autoimmunity, does low-dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J Obstet Gynaecol Res. 2015 May;41(5):722-8.
- Ott J, Pecnik P, Promberger R, Pils S, Seemann R, Hermann M, Frigo P. Dehydroepiandrosterone in women with premature ovarian failure and Hashimoto’s thyroiditis. Climacteric. 2014 Feb;17(1):92-6.
- Solerte SB, Precerutti S, Gazzaruso C, Locatelli E, Zamboni M, Schifino N, Bonacasa R, Rondanelli M, Taccani D, Ferrari E, Fioravanti M. Defect of a subpopulation of natural killer immune cells in Graves’ disease and Hashimoto’s thyroiditis: normalizing effect of dehydroepiandrosterone sulfate. Eur J Endocrinol. 2005 May;152(5):703-12.
- Krysiak R, Kowalcze K, Okopień B. The effect of testosterone on thyroid autoimmunity in euthyroid men with Hashimoto’s thyroiditis and low testosterone levels. J Clin Pharm Ther. 2019 Oct;44(5):742-749.
- Ansar Ahmed S, Young PR, Penhale WJ. Beneficial effect of testosterone in the treatment of chronic autoimmune thyroiditis in rats. J Immunol. 1986 Jan;136(1):143-7.
- Ahmed SA, Penhale WJ. The influence of testosterone on the development of autoimmune thyroiditis in thymectomized and irradiated rats. Clin Exp Immunol. 1982 May;48(2):367-74.
- Fässler R, Dietrich H, Krömer G, Böck G, Brezinschek HP, Wick G. The role of testosterone in spontaneous autoimmune thyroiditis of Obese strain (OS) chickens. J Autoimmun. 1988 Feb;1(1):97-108.
- Gause WC, Marsh JA. Effects of testosterone on the development of autoimmune thyroiditis in two strains of chicken. Clin Immunol Immunopathol. 1985 Jul;36(1):10-7.
- Navarro MA, Vidaller A, Bonnín MR, Mitjavila F, Ortolá JB, Moga I, Pac MV. Salivary testosterone during the menstrual cycle in women with systemic lupus erythematosus. Lupus. 1997;6(5):484-5.
- Macció DR, Calfa G, Roth GA. Oral testosterone in male rats and the development of experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 2005;12(4):246-54. PubMed PMID: 15990455.
- Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997 Jul 1;159(1):3-6.
- Ziehn MO, Avedisian AA, Dervin SM, Umeda EA, O’Dell TJ, Voskuhl RR. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci. 2012 Sep 5;32(36):12312-24.
- Heiry M, Afra P, Matsuo F, Greenlee JE, Clardy SL. Improvement of GAD65-associated autoimmune epilepsy with testosterone replacement therapy. Neurol Neuroimmunol Neuroinflamm. 2015 Aug 13;2(5):e142.
- Frye C. Hormonal influences on seizures: basic neurobiology. Int Rev Neurobiol 2008;83:27–77.
- Frye C. The role of androgens in epilepsy. Expert Rev Neurother 2006;6:1061–76
- Herzog AG. Psychoneuroendocrine aspects of temporolimbic epilepsy. Part II: epilepsy and reproductive steroids. Psychosomatics 1999;40:102–8.
- Bizzarro A, Valentini G, Di Martino G, DaPonte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter’s syndrome. J Clin Endocrinol Metab. 1987 Jan;64(1):32-6.
- Vendramini AC, Soo C, Sullivan DA. Testosterone-induced suppression of autoimmune disease in lacrimal tissue of a mouse model (NZB/NZW F1) of Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):3002-6.
- Ocon A, Peredo-Wende R, Kremer JM, Bhatt BD. Significant symptomatic improvement of subacute cutaneous lupus after testosterone therapy in a female-to-male transgender subject. Lupus. 2018 Feb;27(2):347-348.
- Gordon C, Wallace DJ, Shinada S, Kalunian KC, Forbess L, Braunstein GD, Weisman MH. Testosterone patches in the management of patients with mild/moderate systemic lupus erythematosus. Rheumatology (Oxford). 2008 Mar;47(3):334-8.
- Kanda N, Tsuchida T, Tamaki K. Testosterone suppresses anti-DNA antibody production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1703-11.
- Olsen NJ, Kovacs WJ. Case report: testosterone treatment of systemic lupus erythematosus in a patient with Klinefelter’s syndrome. Am J Med Sci. 1995 Oct;310(4):158-60.
- Bizzarro A, Valentini G, Di Martino G, DaPonte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter’s syndrome. J Clin Endocrinol Metab. 1987 Jan;64(1):32-6.
- Costello MJ, Singer JI. Lupus erythematosus, chronic disseminated type, showing a dramatic response to testosterone therapy. AMA Arch Derm Syphilol. 1952 May;65(5):631-2.
- Verheul HA, Deckers GH, Schuurs AH. Effects of nandrolone decanoate or testosterone decanoate on murine lupus: further evidence for a dissociation of autoimmunosuppressive and endocrine effects. Immunopharmacology. 1986 Apr;11(2):93-9.
- Verheul HA, Stimson WH, den Hollander FC, Schuurs AH. The effects of nandrolone, testosterone and their decanoate esters on murine lupus. Clin Exp Immunol. 1981 Apr;44(1):11-7.
- Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011 May 1;186(9):5162-72.
- Haider A, Gooren LJ, Padungtod P, Saad F. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalisation of plasma testosterone levels in hypogonadal elderly men. Andrologia. 2009 Feb;41(1):7-13.
- Morton JI, Weyant DA, Siegel BV, Golding B. Androgen sensitivity and autoimmune disease. I. Influence of sex and testosterone on the humoral immune response of autoimmune and non-autoimmune mouse strains to sheep erythrocytes. Immunology. 1981 Dec;44(4):661-9.
- Burek CL, Rose NR. Cell-mediated immunity in autoimmune thyroid disease. Hum Pathol. 1986 Mar;17(3):246-53.
- Imam S, Dar P, Paparodis R, Almotah K, Al-Khudhair A, Hasan SA, Salim N, Jaume JC. Nature of coexisting thyroid autoimmune disease determines success or failure of tumor immunity in thyroid cancer. J Immunother Cancer. 2019 Jan 7;7(1):3.
- Solerte SB, Precerutti S, Gazzaruso C, Locatelli E, Zamboni M, Schifino N, Bonacasa R, Rondanelli M, Taccani D, Ferrari E, Fioravanti M. Defect of a subpopulation of natural killer immune cells in Graves’ disease and Hashimoto’s thyroiditis: normalizing effect of dehydroepiandrosterone sulfate. Eur J Endocrinol. 2005 May;152(5):703-12
- Harii N, Lewis CJ, Vasko V, et al. Thyrocytes express a functional toll-like receptor 3: overexpression can be induced by viral infection and reversed by phenylmethimazole and is associated with Hashimoto’s autoimmune thyroiditis. Mol Endocrinol. 2005;19:1231–1250
- Morohoshi K, Takahashi Y, Mori K. Viral infection and innate pattern recognition receptors in induction of Hashimoto’s thyroiditis. Discov Med. 2011 Dec;12(67):505-11.
- Mori K, Yoshida K. Viral infection in induction of Hashimoto’s thyroiditis: a key player or just a bystander? Curr Opin Endocrinol Diabetes Obes. 2010 Oct;17(5):418-24.
- Vrbikova J, Janatkova I, Zamrazil V, Tomiska F, Fucikova T. Epstein-Barr virus serology in patients with autoimmune thyroiditis. Exp Clin Endocrinol Diabetes. 1996;104(1):89-92.
- Thomas D, Karachaliou F, Kallergi K, et al. Herpes virus antibodies seroprevalence in children with autoimmune thyroid disease. Endocrine. 2008;33:171–175. The seroprevalence of EBV infection was reported to be higher in children with autoimmune thyroid disorders when compared to the controls
- Janegova A, Janega P, Rychly B, Kuracinova K, Babal P. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66(2):132-6.
- Nagata K, Okuno K, Ochi M, Kumata K, Sano H, Yoneda N, Ueyama J, Matsushita M, Kuwamoto S, Kato M, Murakami I, Kanzaki S, Hayashi K. Production of thyrotropin receptor antibodies in acute phase of infectious mononucleosis due to Epstein-Barr virus primary infection: a case report of a child. Springerplus. 2015;4:456.)
- Tozzoli R, Barzilai O, Ram M, Villalta D, Bizzaro N, Sherer Y, Shoenfeld Y.. Infections and autoimmune thyroid diseases: parallel detection of antibodies against pathogens with proteomic technology. Autoimmun Rev. 2008;8:112–115.
- Dittfeld A, Gwizdek K, Michalski M, Wojnicz R. A possible link between the Epstein-Barr virus infection and autoimmune thyroid disorders. Cent Eur J Immunol. 2016;41(3):297-301.
- Thomas D, Liakos V, Michou V, Kapranos N, Kaltsas G, Tsilivakos V, Tsatsoulis A. Detection of herpes virus DNA in post-operative thyroid tissue specimens of patients with autoimmune thyroid disease. Exp Clin Endocrinol Diabetes. 2008;116:35–39.
- Di Crescenzo V, D’Antonio A, Tonacchera M, Carlomagno C, Vitale M. Human herpes virus associated with Hashimoto’s thyroiditis. Infez Med. 2013 Sep;21(3):224-8.
- Serranti D, Indolfi G, Nebbia G, Cananzi M, D’Antiga L, Ricci S, Stagi S, Azzari C, Resti M; Italian Study Group for Treatment of Chronic Hepatitis C in Children. Transient hypothyroidism and autoimmune thyroiditis in children with chronic hepatitis c treated with pegylated-interferon-α-2b and ribavirin. Pediatr Infect Dis J. 2018 Apr;37(4):287-291.
- Lehmann HW, Lutterbüse N, Plentz A, Akkurt I, Albers N, Hauffa BP, Hiort O, Schoenau E, Modrow S. Association of parvovirus B19 infection and Hashimoto’s thyroiditis in children. Viral Immunol. 2008 Sep;21(3):379-83.
- Kawai H, Inui T, Kashiwagi S, et al. HTLV-I infection in patients with autoimmune thyroiditis (Hashimoto’s thyroiditis) J Med Virol. 1992;38:138–141.
- Akamine H, Takasu N, Komiya I, et al. Association of HTLV-I with autoimmune thyroiditis in patients with adult T-cell leukaemia (ATL) and in HTLV-I carriers. Clin Endocrinol (Oxf) 1996;45:461–466.
- Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5.
- Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329–337.
- Drabick JJ, Horning VL, Lennox JL, Coyne PE, Oster CN, Knight RD, Dillard TA, Fuller SA, Damato JJ, Burke DS. A retrospective analysis of diseases associated with indeterminate HIV western blot patterns. Mil Med. 1991;156:93–96.
- He Z, Wang T, Shan D, Zhang X, Wang G, Wang F. [Detection and analysis of serum level of anti-Candida enolase IgG antibody in patients with autoimmune diseases]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 Aug;33(8):1113-1117.
- Gürsoy S, Koçkar T, Atik SU, Önal Z, Önal H, Adal E. Autoimmunity and intestinal colonization by Candida albicans in patients with type 1 diabetes at the time of the diagnosis. Korean J Pediatr. 2018 Jul;61(7):217-220.
- Fraga-Silva TF, Mimura LA, Marchetti CM, Chiuso-Minicucci F, França TG, Zorzella-Pezavento SF, Venturini J, Arruda MS, Sartori A. Experimental autoimmune encephalomyelitis development is aggravated by Candida albicans infection. J Immunol Res. 2015;2015:635052.
- Benvenga S, Santarpia L, Trimarchi F, Guarneri F. Human thyroid autoantigens and proteins of Yersinia and Borrelia share amino acid sequence homology that includes binding motifs to HLA-DR molecules and T-cell receptor. Thyroid. 2006 Mar;16(3):225-36.
- Benvenga S, Guarneri F, Vaccaro M, Santarpia L, Trimarchi F. Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid. 2004 Nov;14(11):964-6
- Tozzoli R, Barzilai O, Ram M, et al. Infections and autoimmune thyroid diseases: parallel detection of antibodies against pathogens with proteomic technology. Autoimmun Rev. 2008;8:112–115.
- Ingram KG, Crouch DA, Douez DL, Croy BA, Woodward B. Effects of triiodothyronine supplements on splenic natural killer cells in malnourished weanling mice. Int J Immunopharmacol. 1995 Jan;17(1):21-32.
- Provinciali M, Muzzioli M, Fabris N. Thyroxine-dependent modulation of natural killer activity. J Exp Pathol. 1987 Summer;3(4):617-22.
- Sharma SD, Tsai V, Proffitt MR. Enhancement of mouse natural killer cell activity by thyroxine. Cell Immunol. 1982 Oct;73(1):83-97.
- Gala RR. Influence of thyroxine and thyroxine with growth hormone and prolactin on splenocyte subsets and on the expression of interleukin-2 and prolactin receptors on splenocyte subsets of Snell dwarf mice. Proc Soc Exp Biol Med. 1995 Nov;210(2):117-25.
- Marsh JA, Johnson BE, Lillehoj HS, Scanes CG. Effect of thyroxine and chicken growth hormone on immune function in autoimmune thyroiditis (obese) strain chicks. Proc Soc Exp Biol Med. 1992 Jan;199(1):114-22.
- Tomazic VJ, Novotny EA, Ordonez JV. Thymosin alpha 1-induced modulation of cellular responses and functional T-cell subsets in mice with experimental autoimmune thyroiditis. Cell Immunol. 1985 Jul;93(2):340-9.
- Tomazic V, Suter CM, Chretien PB. Experimental autoimmune thyroiditis: modulation of the disease level in high and low responder mice by thymosin. Clin Exp Immunol. 1984 Oct;58(1):83-9.
- Pica F, Mastino A, Grelli S, Jezzi T, Favalli C. Combined treatment with thymosin alpha 1 and interferon enhances NK activity in immunosuppressed tumor bearing mice. J Chemother. 1989 Jul;1(4 Suppl):1167-9.
- Umeda Y, Sakamoto A, Nakamura J, Ishitsuka H, Yagi Y. Thymosin alpha 1 restores NK-cell activity and prevents tumor progression in mice immunosuppressed by cytostatics or X-rays. Cancer Immunol Immunother. 1983;15(2):78-83.
- Favalli C, Mastino A, Jezzi T, Grelli S, Goldstein AL, Garaci E. Synergistic effect of thymosin alpha 1 and alpha beta-interferon on NK activity in tumor-bearing mice. Int J Immunopharmacol. 1989;11(5):443-50.
- Matteucci C, Minutolo A, Pollicita M, Balestrieri E, Grelli S, D’Ettorre G, Vullo V, Bucci I, Luchini A, Aquaro S, Sinibaldi-Vallebona P, Macchi B, Perno CF, Mastino A, Garaci E. Thymosin α 1 potentiates the release by CD8(+) cells of soluble factors able to inhibit HIV-1 and human T lymphotropic virus 1 infection in vitro. Expert Opin Biol Ther. 2015;15 Suppl 1:S83-100.
- Buzoianu IC, Arghir OC, Circo E. [Smoking and chronic autoimmune thyroiditis]. Pneumologia. 2010 Oct-Dec;59(4):211-4.
- Galanti MR, Cnattingius S, Granath F, Ekbom-Schnell A, Ekbom A. Smoking and environmental iodine as risk factors for thyroiditis among parous women. Eur J Epidemiol. 2007;22(7):467-72
- Rasic-Milutinovic Z, Jovanovic D, Bogdanovic G, Trifunovic J, Mutic J. Potential Influence of Selenium, Copper, Zinc and Cadmium on L-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp Clin Endocrinol Diabetes. 2017 Feb;125(2):79-85.
- Pacini F, Vorontsova T, Molinaro E, Kuchinskaya E, Agate L, Shavrova E, Astachova L, Chiovato L, Pinchera A. Prevalence of thyroid autoantibodies in children and adolescents from Belarus exposed to the Chernobyl radioactive fallout. Lancet. 1998 Sep 5;352(9130):763-6.
- Agate L, Mariotti S, Elisei R, Mossa P, Pacini F, Molinaro E, Grasso L, Masserini L, Mokhort T, Vorontsova T, Arynchyn A, Tronko MD, Tsyb A, Feldt-Rasmussen U, Juul A, Pinchera A. Thyroid autoantibodies and thyroid function in subjects exposed to Chernobyl fallout during childhood: evidence for a transient radiation-induced elevation of serum thyroid antibodies without an increase in thyroid autoimmune disease. J Clin Endocrinol Metab. 2008 Jul;93(7):2729-36.
- Schell LM, Gallo MV, Ravenscroft J, DeCaprio AP. Persistent organic pollutants and anti-thyroid peroxidase levels in Akwesasne Mohawk young adults. Environ Res. 2009 Jan;109(1):86-92