By León F. Villegas Vílchez,1,2 Julio Hidalgo Ascencios,2 and Thomas P. Dooley3
This article is reprinted with the consent of the authors as a redacted summary of the following: León F. Villegas Vílchez, Julio Hidalgo Ascencios, and Thomas P. Dooley. GlucoMedix®, an extract of Stevia rebaudiana and Uncaria tomentosa, reduces hyperglycemia, hyperlipidemia, and hypertension in rat models without toxicity: a treatment for metabolic syndrome. BMC Complementary Medicine and Therapies. (2022) 22:62; https//doi.org/10.1186/s12906-022-03538-9
Abstract
Background: The objective of this in vivo study is to evaluate in five rat models the pharmacologic effects and toxicity of a commercial hydro-alcoholic extract, GlucoMedix®, derived from Stevia rebaudiana and the pentacyclic chemotype of Uncaria tomentosa (Willd.) DC, for use as a treatment for metabolic syndrome. The extract contains phytochemicals of Stevia (e.g., steviol glycosides) and Uncaria (e.g., pentacyclic oxindole alkaloids, but lacks tetracyclic oxindole alkaloids).
Methods: The pharmacologic assessments in three rat models include reductions in chemically induced hyperglycemia, hyperlipidemia (cholesterol and triglycerides), and hypertension, all of which are comorbidities of metabolic syndrome. Acute toxicity and 28-day subacute toxicity were assessed in rat models at doses higher than those used in the efficacy models.
Results: The acute oral toxicity was evaluated in Holtzman rats and the extract did not produce acute toxic effects or lethality, with the LD50 >5000 mg/kg (extract wet weight). Furthermore, subacute oral toxicity was evaluated in rats for 28 days at daily doses as high as 2000 mg/kg without toxicity or abnormal clinical chemistry or hematological effects. Daily oral doses of 250-1000 mg/kg were used to evaluate the treatment effects in hyperglycemic (alloxan-induced and glibenclamide-controlled), hyperlipidemic (cholesterol-induced and atorvastatin-controlled), and hypertensive (L-NAME-induced and enalapril-controlled) rat models. Alloxan-induced hyperglycemia was reduced in a dose-dependent manner within 28 days or less. Cholesterol-induced hyperlipidemic rats exhibited dose-dependent reductions in cholesterol and triglycerides at 21 days. Furthermore, GlucoMedix® produced a dose-dependent decrease in systolic and diastolic arterial blood pressure in L-NAME-induced hypertensive rats at 28 days.
Conclusions: The five in vivo rat models revealed that the all-natural phytotherapy GlucoMedix® is a safe and effective treatment for hyperglycemia, hyperlipidemia, and hypertension. This extract is expected to affect multiple comorbidities of metabolic syndrome, without any acute or subacute oral toxicity in humans. Although multiple prescription drugs are well known for the treatment of individual comorbidities of metabolic syndrome, no drug monotherapy concurrently treats all three comorbidities.
Results: Pharmacologic Dose Responses
In Figure 8 (below) the dose responses of the various rat efficacy models are summarized. The treatment effects of GlucoMedix® are expressed as the percentage of the chemically induced maximum levels minus the uninduced baseline levels in each animal model. Herein, 100% represents no inhibition of the induced parameter, whereas 0% is total inhibition of the induced parameter (i.e., reduction to baseline). The glucose result is from 28 days of treatment; the cholesterol and triglycerides are from 21 days; and the blood pressures are from 28 days. It is unknown whether any further reductions in cholesterol and/or triglycerides would be achieved by an additional week of treatment (i.e., from 21 to 28 days).
Dose response curves are evident for each of the animal models tested, with the anti-hyperglycemic effect of GlucoMedix® being the most potent when comparing the three independent animal models. Note within the prior three sections (above) the relative effectiveness of the highest dose of GlucoMedix® vs. the three pharmaceutical positive controls – Enalapril, Atorvastatin, and Glibenclamide. Even at the lowest oral dose of 250 mg/kg of the Uncaria plus Stevia extract, there is evidence of reductions in blood pressure, lipids, and glucose.
If Inhibitory Concentration 50% (IC50) values are applied to GlucoMedix®, then the IC50 values for glucose, cholesterol, and triglycerides in these selected rat models are below 250 mg/kg, and for mean blood pressure it is approximately 500 mg/kg. Thus, to achieve a minimum of half-maximal inhibition in a genetic or diet-induced rat model of metabolic syndrome per se (i.e., manifesting multiple comorbid conditions) and for all of the endpoints assessed herein in individual rat models, then the recommended dose is 500 mg/kg. If total pharmacologic blockade is desired (and in this duration of treatment), then the Inhibitory Concentration 100% (IC100) values in the selected rat models herein would be approximately 1,000 mg/kg for glucose, and greater than 1,000 mg/kg for cholesterol, triglycerides, and mean BP. Also, note that 1,000 mg/kg dosing is comparable to the clinical effect of the pharmaceutical positive controls. Furthermore, these IC50 values provide guidance toward allometrically scaled starting oral dosing in humans (see Discussion below).
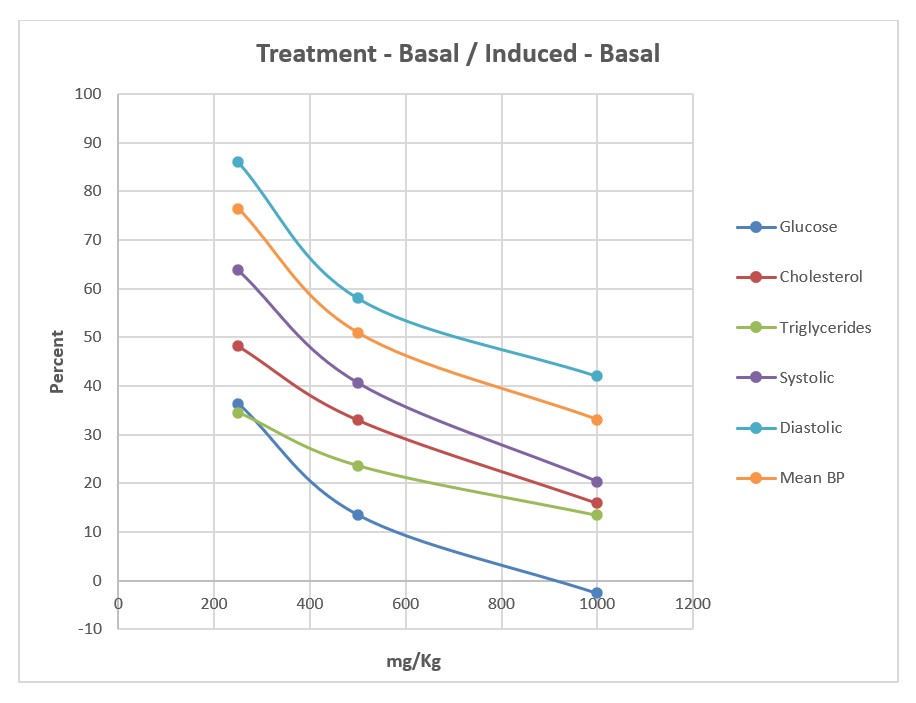
GlucoMedix® (250, 500, 1,000 mg/kg) treatment effects, expressed as the percentage of the chemically induced maximum minus the uninduced baseline; glucose at 28 days; cholesterol and triglycerides at 21 days; systolic, diastolic, and mean BP at 28 days.
Discussion
GlucoMedix® does not produce acute toxic effects in rats; the LD50 being greater than 5.0 g/Kg. Also, in 28-day subacute toxicity studies we did not observe mortality or signs of toxicity, and no significant weight loss was registered. Therefore, the NOAEL for the subacute toxicity study was 2,000 mg/kg. According to the dosage levels evaluated in the subacute and acute toxicity studies, the LOAEL (Lowest Observed Adverse Effect Level) was not found. The only statistically significant effects in the 28-day oral treatments were minor increases in hematocrit, hemoglobin, and red blood cells in males and hematocrit in females. Thus, GlucoMedix® could be considered with a wide margin of safety for oral use in humans.
Regarding efficacy in three animal models, GlucoMedix® reduced the systolic and diastolic arterial pressure in hypertensive animals, which was induced by L-NAME, as evidenced with a 28-day treatment. In hyperglycemic and hyperlipidemic animals treated with GlucoMedix®, substantial and statistically significant beneficial effects were observed. All three rodent efficacy models manifested potent and dose dependent effects at 250-1000 mg/kg (extract wet weight), thus demonstrating pharmacologic benefits without any coincident adverse toxicities. The highest dose (1,000 mg/kg) was comparable to the pharmaceutical positive controls.
Various pharmacologic mechanisms of action (MOAs) of GlucoMedix® are plausible for reducing glucose, lipids, triglycerides, and blood pressure in the rat animal models.
Stevia and steviol glycosides might down-regulate the levels of glucose and lipids in blood, as well as arterial hypertension. Stevia phytochemicals or steviol glycosides were known in human clinical trials to affecttype 2 diabetes.1,2 There is evidence of a possible benefit regarding hypertension in humans.3 However, another study of only 7 patients per group yielded a negative result for hypertension,4 although statistically significant reductions in cholesterol, LDL, and glucose were observed.
The Stevia-derived ingredients were also effective in rat models inalloxan-induced hyperglycemia,5-7 streptozotocin-induced hyperglycemia,8and cholesterol-induced hyperlipidemia.9 Another rat study showed that stevioside and powdered Stevia leaves in high-carbohydrate and high-fat diets caused a significant reduction in blood glucose level after 4 weeks of treatment.10 Our studies in three rat efficacy models are consistent with these prior findings, presuming that the steviol glycosides are contributing to the overall efficacy of GlucoMedix®.
Uncaria extracts have been found to reduce glucose levels in mice and rat animal models.11,12 A hydro-alcoholic extract of Uncaria containing POAs (29.1 mg/g) in a streptozotocin-induced mouse model, showed a reduction in glycemic levels.11 Likewise, rats treated with 75 and 150 mg/kg of Uncaria tomentosa dry extract showed a reduction in blood glucose.12 One possible MOA for this glucose down-regulation is explained by alpha-glucosidase and alpha-amylase inhibitory activities within Uncaria extracts.13,14 These enzymes catalyze the hydrolysis of complex polysaccharides, such as dietary starch and endogenous glycogen. This enzymatic antagonism of biodegradation of polysaccharide precursors might reduce blood glucose, and thus possibly contribute to the overall glycemic regulatory efficacy of GlucoMedix®.
Steviol glycosides and/or Uncaria phytochemicals might be affecting the endocrine and/or neuro-endocrine system, and in particular the hypothalamic-pituitary-adrenal (HPA) axis. Cortisol levels might be a possible mediator under the influence of these bioactive compounds. Cortisol is known to play a key role in glucose utilization. Patients with metabolic syndrome exhibit elevated HPA axis properties leading to hypercortisolism.15,16 Future studies of GlucoMedix® could assess levels of cortisol and insulin.
Another possible MOA is that the Uncaria POAs are affecting the immune system.11,17-19 However, it should be noted that the subacute toxicology study at doses as high as 2000 mg/kg for four weeks did not reveal any significant alterations in white blood cell numbers or ratios. If the MOA is immunomodulatory, it is not being achieved by altering the number of white blood cells.
Regardless of the MOA, one significant factor to consider is that the three rodent efficacy models involved experimental induction agents (i.e., alloxan, L-NAME, and cholesterol) that result in parameters exceeding normal physiologic levels, whereas the acute and subacute toxicology models were not dependent on any induction events. In other words, the toxicity model was performed in a natural physiologic state. The 28-day toxicity studies further underscore that any efficacy benefit in hyper-normal physiological states (e.g., induced states or disease states) is not expected to result in any adverse outcome extending below baseline parameters in normal laboratory animals (or humans).
A beneficial aspect of these animal model efficacy and toxicity studies run in parallel is the establishment of a favorable therapeutic index. In other words, GlucoMedix® achieved the desired efficacy endpoints without any observable toxicity at or above the effective dose(s) and at coincident time points (i.e., at 3-4 weeks).
Toxicologic studies in rodents have demonstrated the safety of extracts and isolated compounds of Uncaria tomentosa and Stevia rebaudiana.7, 20, 21 Our study shows that GlucoMedix® has an LD50 in rats greater than 5,000 mg/kg of body weight, and well-being parameters such as sleep, behavior pattern, motor activity, skin, coat, and appetite were normal. No weight loss was observed after two weeks of observation.
Metabolic syndrome is often associated with type 2 diabetes, but it can exist in patients lacking this comorbidity. In the US a diagnosis typically involves any three of five comorbidities, as per the NCEP-ATP III criteria. Although type 2 diabetes is common, it is not the essential factor driving the pathophysiology of metabolic syndrome in all patients.
Although some articles assert that alloxan induction is an experimental model for type 2 diabetes,5,6 it should be noted that the alloxan-induced and glibenclamide-controlled rat model is more closely related to type 1 diabetes (insulin insufficiency), rather than type 2 diabetes (insulin resistance). This suggests that GlucoMedix® might be stimulating production of insulin from the remaining pancreatic beta cells following toxic damage to the tissue by alloxan. Thus, this animal model does not provide a precise correlate for type 2 diabetes within metabolic syndrome. Beyond the scope of the present experiment, two relevant rodent models for consideration for future confirmatory studies are C57BL6J male mice on high fat diet and Zucker Diabetic Fatty (ZDF) rats.22,24
Stevia extract has long been used for the treatment of diabetes in South America.25 Furthermore, stevioside is a potent sweetener with no calories. Thus, Stevia-derived products can achieve reductions in blood glucose in humans by two means: (a) as a substitute for dietary sugars, thus reducing ingested sugars; and (b) as a pharmacologic active ingredient affecting glucose homeostasis.
The GlucoMedix® extract of Uncaria and Stevia shows anti-hyperglycemic activity in alloxan-induced rats treated at doses of 250-1000 mg/Kg of body weight. GlucoMedix® might regulate the level of glucose by increasing insulin secretion and/or by a better utilization of glucose by peripheral tissues and muscles in diabetic rats.
One of the most common complications of diabetes mellitus is cardiovascular disease. Other studies have suggested that Uncaria, Stevia, and their metabolites promote cardiovascular health and reduce hypertension. Our results with GlucoMedix® show a decrease in cholesterol and triglyceride levels in hyperlipidemic rats at 21 days and a decrease in blood pressure induced by L-NAME in hypertensive rats at 28 days of treatment with doses of 250-1000 mg/Kg.
The 1,000 mg/kg daily dose (wet weight) is equivalent to administering 81.8 mg of steviol glycosides, 16.98 ug of isopteropodine, and 4.71 ug of pteropodine per kg of body weight in rats. This maximum tested dose of GlucoMedix® displayed the same or similar potency to the three pharmaceutical positive controls. However, comparison of dosing to other published rodent models treated with other extracts is somewhat problematic. For example, Ahmad and coworkers demonstrated anti-hyperlipidemic effects in cholesterol-induced rats using 200-500 parts per million of Stevia extract; presumably this represents 200-500 mg/kg dry weight of Stevia powder.9 Thus, they tested 200-500 mg/kg vs. 29.2-116.7 mg/kg of Stevia powder within GlucoMedix® in the present study. As another example, Kujur and coworkers demonstrated anti-hyperglycemic effects in alloxan-induced rats using 50-100 mg/kg (wet weight) of Stevia extracts at 28 days; the dry weights are unknown.7
If the 250, 500, and 1,000 mg/Kg daily doses in rats (ca. 0.24 Kg) are extrapolated via allometric dosage conversion for oral administration in humans (65 Kg and 0.75 exponent), the corresponding allometric daily doses of GlucoMedix® required in an adult for “similar” pharmacologic effects would be 4, 8, and 16 g (wet weight).
Given that the extract mixture contains ca. 2.56 mg/100 ml of POAs, then these human allometric doses would contain only 0.10, 0.20, and 0.41 mg of POAs. Note that few active pharmaceutical ingredients (APIs) in the pharmacopeia are effective in the sub-milligram level in human adults. However, predicate examples do exist; an example is the phytochemical scopolamine that is effective at 0.1-0.5 mg in humans.26,27 If the POAs are contributing to the efficacy endpoints, then they would by necessity be highly potent phytochemicals.
Allometric dosage conversion presumes similarities between the two species regarding pathophysiology, pharmacokinetics, and pharmacodynamics. A suggested human starting oral daily dose of GlucoMedix® that might be effective within 4 weeks at treating metabolic syndrome or its comorbidities is 4 g. Given that the IC50 values for glucose and lipids (cholesterol and triglycerides) were below 250 mg/kg in rats, then it is reasonable to speculate that adult doses lower than 4 g (and/or with longer duration of treatment) might also be effective in humans.
A physician-sponsored retrospective case series study has been reported of six humans afflicted by type 2 diabetes, which were treated with GlucoMedix® at daily doses of 4 or 6 g (28). The patients experienced reductions in hyperglycemia, and several of them coincidentally reduced or ceased treatments with prescription drugs or insulin. Thus, the suggested minimum allometric dose (4 g) based upon the rat efficacy model for hyperglycemia coincides with the minimum dosage used within the type 2 diabetes case series.
Furthermore, based upon the relative potencies in rats across the three indications summarized within Figure 8, the most potent effect was observed for hyperglycemia (IC50 <250 mg/kg), followed by hyperlipidemia (IC50 <250 mg/kg), and finally mean blood pressure (IC50 ~500 mg/kg). If this correlation also applies in humans, it suggests that treatment of hypertension might require higher daily dosing with GlucoMedix® than is required for glucose regulation.
Conclusions
Limitations of this work should be noted: (a) The pharmacologic effects might be due to Stevia alone, Uncaria alone, or the combination thereof; (b) The efficacy studies were based upon established chemical induction models, rather than genetic disease models that are predisposed to diabetes, hyperlipidemia, and hypertension. Future studies could also focus on alternative rodent models for hyperglycemia (and obesity) that mirror type 2 diabetes, such as C57BL6J male mice on high fat diet or Zucker Diabetic Fatty (ZDF) rats; and (c) The beneficial pharmacologic effects and lack of toxicity were assessed for up to 21 days (anti-hyperlipidemia) or 28 days (anti-hyperglycemia, anti-hypertension, and subacute toxicity).
A safe and effective natural product, such as GlucoMedix®, that can address multiple comorbidities of metabolic syndrome would be a welcome addition to the pharmacopeia and marketplace. We are unaware of any single US FDA-approved drug that can address all three conditions, although inexpensive monotherapies are available for treating hypertension (e.g., ACE inhibitors and beta blockers), hyperlipidemia (e.g., statins), and type II diabetes (e.g., metformin). A physician-sponsored case series of six type 2 diabetic patients suggests that this natural product at 1-1.5x of the suggested starting allometrically-scaled dose can address at least one of the three indications, namely hyperglycemia.28
1 Department of Cellular and Molecular Sciences, Section of Pharmaceutical Sciences, Faculty of Sciences and Philosophy, Universidad Peruana Cayetano Heredia, Lima, Peru
2 Quality Control Service, Research and Development Laboratories, Universidad Peruana Cayetano Heredia, Lima, Peru
3 Research and Development, LivFul Inc., Cheshire, UK (corresponding author)











