By Garth L. Nicolson, PhD, MD (H)1* and Paul C. Breeding, DC2
Abstract
We sought to determine if oral dietary wafers containing a combination of membrane glycerolphospholipids (NTFactor Lipids® wafers, 4-6 g per day) could reduce self-reported peripheral and widespread pain, fatigue, and gastrointestinal symptoms in chronic illness patients. This followed an open label clinical study where the membrane glycerolphospholipid supplement was tested in fibromyalgia patients who had widespread pain and suffered from chronic fatigue and other symptoms. In that clinical study middle-aged fibromyalgia patients showed significant reductions in self-reported pain, fatigue, and gastrointestinal symptoms, and improvements in quality of life (QOL) indicators within one week of taking 4.8 g per day NTFactor Lipids®. As we found in previous cross-over clinical studies, these improvements were dependent on patients’ continuing to take the supplement. Increasing the dose of the membrane glycerolphospholipid supplement to approximately 6 g per day in patients with severe, intractable pain resulted in better resolution of pain and other signs and symptoms compared to lower daily doses, as long as patients continued to take the oral supplement.
Introduction
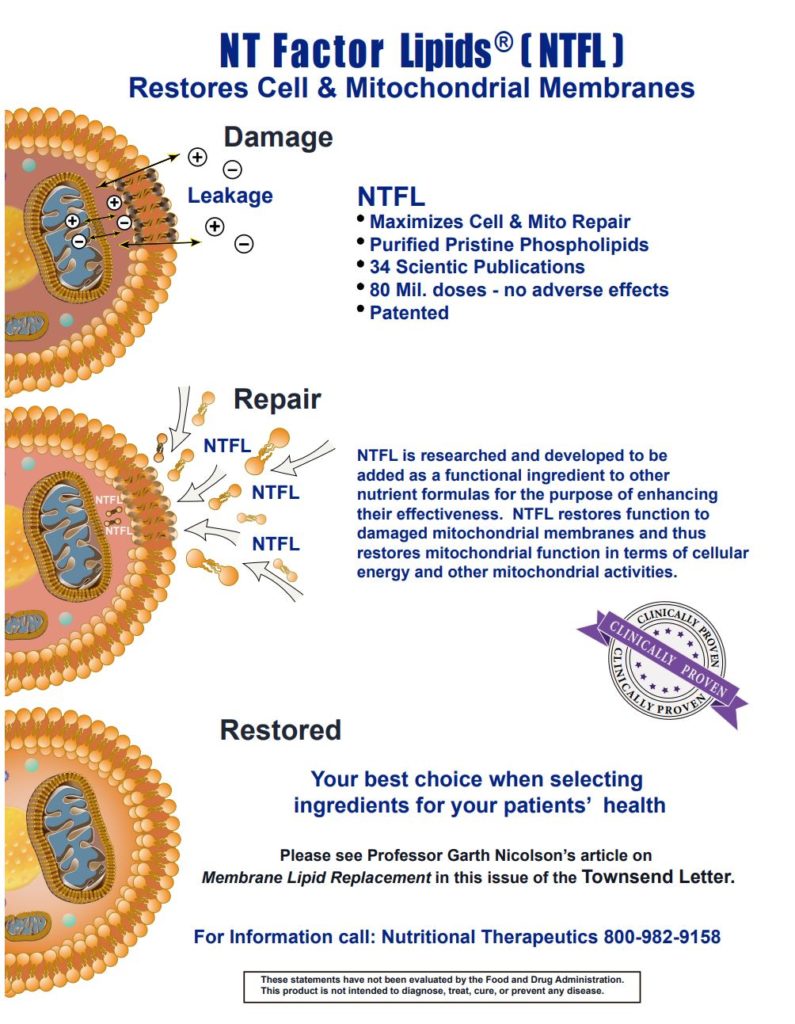
Membrane Lipid Replacement (MLR) using dietary NTFactor Lipids® results in the systemic replacement of damaged cellular membrane glycerolphospholipids with undamaged, unoxidized lipids to ensure the proper function of cellular membranes, including mitochondrial membranes.1,2 By combining the glycerolphospholipids with antioxidants, MLR supplements have proven to be effective in reducing disease-associated symptom severity, age-associated loss of function, and providing organ support.1-3 The MLR supplement NTFactor Lipids® has been utilized in several clinical studies that demonstrate that it significantly reduces fatigue in patients with chronic illnesses and in aged subjects with chronic fatigue and other symptoms.4-7
One of the most common clinical conditions marked by widespread pain and fatigue is fibromyalgia.8,9 This condition is characterized by chronic, widespread pain, abnormal processing of pain, increased sensitivity to external stimuli, fatigue, gastrointestinal symptoms, and changes in memory, mood, and sleep.8-10 Using this criteria it has been estimated that between 0.1-3.3% of the people in Western countries and 2.0% of the United States’ population have fibromyalgia.10 Higher incidence rates of fibromyalgia have been found in females compared to males, and this could be due to differences in hormonal status.9,11
Dietary Supplements
Dietary supplements have been used to reduce symptom severity in patients with fibromyalgia, chronic fatigue and other chronic conditions that have pain and/or fatigue as major symptoms.1-7 Unfortunately, few if any, of these natural supplements have been considered effective in significantly reducing symptom severity, and in maintaining this reduction.12 Some symptoms, such as fatigue and pain, also occur during normal aging, and they are important as secondary symptoms in many if not most chronic diseases.13
Using fibromyalgia patients who had been ill for periods of time greater than six months, we previously found that fatigue was reduced significantly when patients consumed dietary NTFactor Lipids® (2-4 g per day) for 4-8 weeks.4,5 For example, fibromyalgia patients that were placed on 4 g of NTFactor Lipids per day showed approximately 40% reductions in fatigue after eight weeks on NTFactor Lipids.5 In these studies, pain and other symptoms were not recorded, but non-scientific feedback from patients in the study indicated that other symptoms (including pain reported by some patients) were also reduced along with fatigue.
Previously we found that a combination of low-dose controlled-release caffeine (184 mg per day) and 4.8 g per day of NTFactor Lipids® resulted in significant reductions in pain, fatigue, gastrointestinal and other symptoms in fibromyalgia patients within one week.14 Since we found that the significant reductions in fibromyalgia pain and fatigue as well as gastrointestinal symptoms could be achieved with NTFactor Lipids® alone, without any caffeine, follow-on studies have employed only NTFactor Lipids®.15
Case Reports
Recently we published a brief case series that reported that patients with chronic, intractable pain benefited from daily oral supplementation with NTFactor Lipids® (Patented EnergyTM, NTFactor.com).15 This case report was based on a series of patients who had intractable chronic pain but also had other symptoms as well. All of these patients had severe pain. Using an established fibromyalgia symptom severity scale patients scored their pain severity as 0 (no pain), 1 (slight or mild pain), 2 (moderate or considerable pain, often present at a moderate levels), or 3 (severe, continuous pain).10
Case Report 1.15 This patient was a 51-year-old male veteran of the first Gulf War (1991). His symptoms at presentation included severe joint and muscle pain, disabling chronic fatigue, nausea, gastrointestinal symptoms (stomach pain, diarrhea, bloating), intermittent fevers, sleep problems, headaches, and short-term memory impairments that were similar to those of other Persian Gulf war veterans.16,17 Since 1992 these symptoms persisted intermittently without resolution, even though he had been treated for post-traumatic stress disorder.17 He had received diagnoses of chronic fatigue syndrome (CFS) and fibromyalgia in 2005 and had minor surgery in 2015 for benign skin lesions. No further treatments or procedures were conducted, and the patient refused follow-up consultations. Upon presentation in 2017 the patient complained of extreme fatigue, widespread severe musculoskeletal pain, gastrointestinal symptoms, including intermittent stomach pain, abdominal cramps, and bloating, intermittent headaches, sleep difficulties, and an inability to concentrate and retain information. His fibromyalgia symptoms score of 3 on the severity scale included widespread pain. He had stopped all pharmaceutical pain and psychotropic medications but continued to take vitamin and mineral supplements, and he maintained a largely vegetarian diet. Within one week of taking 4.8 g of NTFactor Lipids® wafers, the patient reported significant improvements in pain, fatigue, gastrointestinal problems, headaches, and sleep difficulties (score between 1 and 2). There were also patient-perceived improvements in cognition and short-term memory loss. These improvements have been sustained as long as the patient continues to take the oral glycerolphospholipids.
Case Report 2.15 The patient presented as a 53-year-old female veteran with a congenital bicuspid aortic valve with moderate aortic regurgitation, as revealed by echocardiogram. Previously, an unrelated cardiac electrical issue had resolved with successful cardiac ablation for paroxysmal supra-ventricular tachycardia for A-V re-entry nodal arrhythmia. The patient refused beta-blockers, and this part of her condition resolved within six months. In 2013 she began having sharp stabbing pain and numbness in her feet with tingling and electrical shocks, which progressed up to the thigh area with bilateral muscle weakness in her lower extremities. Cortisone injections failed to give any relief, and neuromas were ruled out with MRI. Sleeping became an issue because of foot pain and tingling sensations. She has maintained supplementation of her largely vegetarian diet with CoQ10, vitamins C, E and B-complex, fish oil, L-Carnitine, curcumin, lipoic acid, liposomal glutathione, and astaxanthin-lutein eye formula. In 2016 she was referred to a neurologist who performed a nerve conduction study and electromyelogram and suggested a spinal MRI, which was refused: diagnosis, idiopathic peripheral neuropathy. She also began having headaches starting at about 4 AM, but refused pharmacological remedies. In 2018 she was diagnosed with posterior vitreous detachment, which subsided after six months. In April 2018 at a fibromyalgia symptom severity score of 3, she started oral NTFactor Lipids® (approximately 2 g per day) wafers and continued the other nutraceuticals as before. Within 3-4 weeks her leg electrical surges/shocks and numbness and tingling had slowly diminished along with her peripheral pain, starting with the thigh area and extending with time down each leg. She continued on the same dose of NTFactor Lipids® for five months and realized that the recommended daily dose of NTFactor Lipids® for her condition was about twice the dose she had been taking, and she also noted that her symptoms would slowly return without the NTFactor Lipids®. After five months she increased the dose of NTFactor Lipids® to 6 g per day, and her condition continued to slowly improve (pain severity of 3 reduced to 1-2). As of 2019 she still had some numbness and very faint tingling in both feet; her chronic headaches are not as severe or frequent, and her sleep difficulties have largely resolved. She has now also added 3-5 mg of liquid melatonin sublingually as well as 20 mg of progesterone cream. Follow-up indicates that these improvements have been sustained, as long as she continues to take the NTFactor Lipids® (6 g per day).