By Blanche D. Grube, DMD, Leslie Douglas, PhD, and Anita Vazquez Tibau
Introduction
Oral health has been separated from whole body health since the mid-1800s, when dental schools became formalized as their own institutions, disconnected from medical schools.1 About the same time, dental and oral sepsis as a cause of disease and its relationship to general medicine became a subject of interest. Several luminaries of the day, like Dr. William Hunter, remarked that oral conditions not only included pyorrhea alveolaris, but stomatitis, and gingivitis of every degree. These conditions were septic in nature and produced pus organisms that were invariably associated with every case of dental caries, even if they seemed insignificant. Dr. Hunter stated, “What the effect had on the person was determined by the individual’s resistance.”2 Then came Dr. Frank Billings who coined the term “focal infection” as “a circumscribed area of tissue infected with pathogenic organisms.”3 Dr. Edwin Rosenow followed Billings and spoke of infections of the teeth, especially multirooted teeth. He found evidence of infection of the pulp, even with asymptomatic teeth. Rosenow concluded that they may be the source of systemic effects and may need to be removed. He stated that root canals should cease.4
Weston Price, DDS, found that root canal teeth were always infected.
No one, however, before or since, has ever done the extensive work on the relationship between root canal teeth and degenerative diseases other than Dr. Weston Price. He founded the research institute originally called the National Dental Association, which became the research section of the American Dental Association. In 1915, he became their first director. During his career, he published over one hundred fifty papers in scientific journals. Throughout his time as director, he led a team of sixty scientists, including leading experts such as Victor Vaughn, president of the American Medical Association; Charles Mayo, the founder of the Mayo Clinic; Milton Rosenau, Professor of Preventive Medicine, Harvard University; Ludwig Hektoen, Professor of Pathology, University of Chicago; Thomas Forsyth, head of the Children’s Dental Infirmary in Boston; and Truman Brophy, Dean of the Chicago College of Dental Surgery. All branches of medicine and dentistry were represented on his research team, including bacteriology, pathology, rheumatology, immunology, chemistry, cardiology, and surgery.5
Dr. Price spent 25 years of his dental career studying and performing research on endodontically treated and pulpless teeth as a continuation of the Focal Infection Theory. Price’s impeccable research on root canal teeth and its causation of many diseases was done by removing the infected tooth from a patient and placing it into a rabbit. Whatever disease the patient had, the rabbit would inevitably develop the same disease symptoms. As a true scientist, Price replicated this protocol thousands of time.5
He found that root canal teeth were always infected, regardless if they were symptomatic or asymptomatic. Dr. Price expanded on this concept and studied root canals, infections, and systemic disease and wrote the two revolutionary books titled, Dental Infections Oral & Systemic and Dental Infections and the Degenerative Diseases.5 By the 1930s, the Focal Infection Theory, which resulted in removal of teeth and tonsils, fell out of favor with some who observed: “’If this craze of violent removal goes on, it will come to pass that we will have a gutless, glandless, toothless – and I am not so sure that we may have, thanks to false psychology and surgery, a witless race.’”6 As stated in the American Journal of Ophthalmology: “’Stripped of tonsils and teeth, often the victim of colonic irrigation, abdominal, and genitourinary operations, the patient may finally be reduced to only those organs necessary for existence, while all the time his ocular disease progresses remorsely to blindness.’”7 Even in the 1930s, some medical doctors were blindsided.
In 1952, the Journal of the American Medical Association published an editorial written by the American Dental Association which stated, “After exerting a tremendous influence on the practice of medicine for a generation, the Theory of Focal Infection in the past 10 to 15 years has fallen in part into disfavor… none of them actually disproves the theory that some foci of infection can in the presence of predisposing or accessory factors produce some systemic diseases.”8
Decades later, Dr. Hal Huggins, author of the ground-breaking book It’s All in Your Head and one of the most outspoken, internationally renowned pioneers of mercury-free dentistry, started what is considered the third amalgam war. Huggins was already a trailblazer in training and educating dentists and health care professionals in biological dentistry, when he started to research oral pathogens. Huggins was a stanch follower of Dr. Weston Price and was keenly aware of the link between the state of the oral cavity, good or bad, and the state of the patient’s overall health. His desire to really get to the root cause of disease prompted him to start his own laboratory, where he developed what was called the “Full View Test,” now known as the Oral Panel offered by DNA Connexions. Based on previous works of Anne Haffajee, Huggins focused on the microbial DNA present in extracted root canal teeth and identified 83 different microbial species of interest. He stated, “Dentists claim they can “sterilize” the tooth before forcing the gutta percha wax down into the canal. Perhaps they can sterilize a column of air in the center of the tooth, but is that really where the problem is? Bacteria wandering out of the dentinal tubules is what Price was finding, and what we were finding in the crushed tooth samples. We tested blood samples adjacent to the removed teeth and analyzed them for the presence of anaerobic bacteria. Approximately 400% more bacteria were found in the blood surrounding the root canal tooth, than were in the tooth itself. It seems that the tooth is the incubator. The periodontal ligament supplies more food, therefore, we saw a higher concentration of bacteria, just outside the root canal tooth.”9
As an educator, Dr. Huggins mentored Dr. Blanche Grube, and together they developed the Huggins-Grube Protocol. The protocol involves restoring the mouth by removing infection and toxic dental materials and replacing them with biocompatible materials. This is referred to as a “Full Dental Revision.” Before his death, Dr. Huggins passed his torch to Dr. Grube. His archives and his laboratory were also taken over by Dr. Grube. She has since changed the name of the lab to DNA Connexions and is expanding various test panels from the original test panels that Dr. Huggins developed. Dr. Grube is also carrying on his legacy by training dentists around the world on the Huggins-Grube Protocol.
Oral Health and Disease
Only recently has the Focal Infection Theory been revisited, and from this, a new area of periodontology has been created, called “periodontal medicine.” This resurgence is due to a plethora of new research in the literature that shows both the direct and indirect impact of periodontal pathogens on overall health. Recent epidemiological, clinical, and experimental studies support the relationship between bacteremia or inflammation due to periodontal disease (PD) and systemic disease.10 Somma et al. described a focal infection as “A localized or generalized infection caused by the dissemination of microorganisms or toxic products from a focus of infection in various organic districts, including the oral district” and stated “Oral inflammatory lesions have been shown unequivocally to contribute to elevated systemic inflammatory responses.”11
The Global Burden of Disease Study (2016) estimated that oral diseases affected half of the world’s population (3.58 billion people) with tooth decay in permanent teeth as the most widespread condition measured. Severe periodontal (gum) disease may result in tooth loss and was estimated to be the 11th most common disease globally.12 A commentary in the Journal of the American Dental Association by Weyant et al discussed The Lancet (2019) journal’s coverage on oral health. This was The Lancet’s first time in their 196-year history that they ever reported on oral health. The authors posed the question, “If this is in fact the ‘tipping point?’” on raising awareness on the long-neglected subject of oral health around the world. They observed that the inequality of the social economics, of dentistry’s availability to all populations, has failed to recognize and improve oral health care globally. This has created a burden on all of society—due to health-related costs, loss of work, etc.—with the most vulnerable populations being the most at risk. The Lancet series is shining a light on oral health and is demanding policy makers to make changes that can only be achieved by uncompromising system transformations of the oral health care systems.13 For example, poor oral health in geriatric patients was investigated in a retrospective observational study to verify if it was a predictor of a mortality risk factor. Their findings showed that there are many diseases of the elderly that are linked to poor oral health and that it increases the risk of hospitalization due to infectious and non-infectious diseases, including in-hospital mortality.14 Oral Manifestations of Systemic Diseases by Rosengard et al. developed an extensive list of general illnesses that are linked to oral health, as listed below.
General Illnesses Linked to Oral Health15
- Ulcerative colitis
- Crohn disease
- Pyostomatitis vegetans
- Gastroesophageal reflux
- Chronic liver disease
- White blood cell disorders
- Leukemias
- Lymphoma
- Cyclic neutropenia
- Langerhans cell histiocytosis
- Multiple myeloma
- Mastocytosis
- Platelet disorders
- Thrombocytopenia
- Red blood cell disorders
- Anemias
- Hemochromatosis
- Congenital erythropoietic porphyria
- Pulmonary Conditions
- Granulomatosis with polyangiitis
- Sarcoidosis
- Multisystem Conditions
- Amyloidosis
- HIV Disease
- Candidiasis
- Herpes simplex
- Hairy leukoplakia
- Kaposi sarcoma
- Cytomegalovirus
- Human papillomavirus
- Aphthous like ulcerations
- Cutaneous Diseases
- Psoriasis
- Acanthosis nigricans
- Neurologic Diseases
- Neurofibromatosis types 1 and 2
- Endocrine Diseases
- Diabetes
- Multiple endocrine neoplasia
- Thyroid disorders
- Parathyroid disorders
- Adrenal disorders
- Hypocorticalism
- Hypercortisolism
- Drug-Induced Conditions
- Aphthous stomatitis
- Dry mouth
- Lichen planus
- Gingival overgrowth (hyperplasia)
- Candidiasis (secondary to inhaled steroids)
What Science Says About Dental Materials, Root Canal Treatment, Risk Factors, and Periodontal Disease
According to the American Association of Endodontists (AAE), bacteria are the major cause of pulpal and periapical diseases. Due to the complexity of the root canal system even with saline irrigants or antibacterial irrigants, none are perfect. Researchers continue to look for a technique or ideal material that can completely clean an infected root canal.16 The Endodontic Treatment Statistics survey (2005-2006), estimated that 22.3 million endodontic procedures were performed in the United States.17 Carlson stated, “In our dental research we demonstrate the hundreds of infected but asymptomatic ‘root canals’ and ‘dental implants’ were in fact obstacles to the innate ‘cleansing process’ inherent in Nature. Overwhelming evidence is presented confirming that ‘modern endodontics’ leaves gangrenous tissues in the human jaw.”18
Gutta-percha has been used as a root canal filling material since its discovery by Edwin Truman in 1847. In 1990, Pascon et al. looked at the cytotoxicity of fourteen commercially available and three experimental brands of gutta-percha. They found that all gutta-percha points tested were toxic and that toxicity was attributed to leakage of zinc ions into the fluids.19 Additionally, there are many other types of root canal sealers, some are resin-based, silicone-based, bioceramic-based, and even mercury amalgam has been used as a root-end filling material. Root canal sealer materials have been studied and have been found to cause DNA damage and also have been shown to be toxic.20 For example, a prospective clinical study on blood mercury levels following endodontic root-end surgery with amalgam was conducted on fourteen patients using a zinc-free amalgam. The sample collection of blood was done three times: immediately before, immediately after, and one week after treatment. After one-week, mercury levels increased in the blood. The research found that while the level of mercury increased significantly after one week, the amalgam retroseal may continue to release mercury causing a threat to the patient. Even though the blood levels were under the toxic mercury threshold, they cautioned to use non-mercury biocompatible root-end filling materials.21

Alkahtani et al. investigated the cytotoxicity of QMixTM endodontic irrigating solution on human bone marrow mesenchymal stem cells to compare it with sodium hypochlorite. They stated that, “an ideal root canal irrigant solution should be non-toxic, with a broad antimicrobial spectrum and the ability to dissolve necrotic pulp tissue, inactivating endotoxins, and either prevent the formation of a smear layer or dissolve it. Currently, no single solution is able to achieve these goals, and the combined, concomitant or sequential use of two or more irrigating solutions is thus required.” They found both solutions tested to be toxic to human bone marrow, but QMixTM was more biocompatible.22
Prada et al. noted that the main cause of endodontic failure is the persistence of microorganisms that cause an intraradicular or extratradicular infection and that become resistant to disinfection measures. These microorganisms have the following similarities, which make them able to evade sterilization: the ability to form a biofilm, to locate in areas unreachable to root canal instrumentation techniques, synergism, and the ability to express survival genes and activate alternative metabolic pathways.23 A study by Yan et al. revealed that the difficulty with root canal treatments is that dentin from root canal teeth presented considerably lower strength, than unrestored teeth. Even though the durability of dentin decreases with age, the dentin of root canal teeth showed more damage.24 It has been reported that vertical root fractures, are the third most common cause of tooth loss, after dental caries and periodontal disease (PD). Additionally, it is these fractures, whether detected or undetected, that give the microorganisms more surface area to colonize. Vertical fractures, are complete or incomplete, starting at the root of a tooth. According to Garcia-Guerrero et al. endodontic treatment had the highest risk factor for vertical root fractures after a one-to-eight-year follow up, noting that 94% of the root fractures, had been endodontically treated.25
Another pervasive problem is apical periodontitis (AP), which is an inflammatory process around the apex of a tooth’s root. This worsens with age, and one in two people over 50 years old, will develop AP.26 Risk factors for developing AP includes root fillings, coronal restorations, primary carious lesions, and reduced marginal bone level and molar teeth.27
There is a connection between specific microorganisms from the oral cavity and various cancers and systemic diseases.
A national study on AP in root-filled teeth was conducted on the 30 year and older population in Finland. The results showed that AP occurred more often in root-filled teeth and more often in men than women. If there was insufficient root filling material during the endodontic treatment in either men or women, the risk for AP doubled.28 The incidence of AP in root canal teeth was also explored in an urban Saudi female population. Of the 1,108 root-canal treated teeth, 813 (73.4%) also presented with AP, noting that the quality of root canal treatment, coronal restoration, and of cast restoration is significantly associated with periapical status in root-filled teeth.29
One hundred fifty dentists in Abidjan, Cote d’Ivoire, West Africa, were sent a survey that included public and private clinics, to determine the incidence of complications arising from endodontic procedures. Of the original questionnaires sent, 135 dentists responded, and almost all of them (94.8%) had faced problems during the treatment. Some problems that were common were fracturing of instruments during exploring of the root canal (72.58%) also during shaping (55.47%), canal wall damage (54.68%), overfill of the obturation (55.47%), and flare-up without swelling often after the procedure (81.49).30A study on 1,085 root canal-treated teeth were randomly selected in a Taiwanese population and were assessed as to the quality of treatment by eight endodontic experts. Some of the results included overfilling, underfilling, adequate filling, and no filling. They found that about 70% of the teeth that were treated had either insufficient filling length or sealing density.31 Lechner et al. investigated the impact of AP in root-filled and endodontically treated teeth and its relationship among healthy controls and patients with systemic diseases. They found the frequency of AP was almost twice as high in the patients with systemic diseases, stating that local pathologies caused by endodontically treated teeth may increase immunological and systemic dysfunction.32

Lin et al. investigated the risk association between unfinished root canal treatments and hospitalization for pneumonia using a nationwide population-based database. The study included 116,490 subjects who received and started a root canal treatment with no history of pneumonia before 2005, and were observed until the end of 2011. During 2005 to 2011, a total of 1,285 subjects were hospitalized for pneumonia. They reported that an unfinished root canal treatment can leave a space for bacterial accumulation, that can leak into the oral cavity, and disseminate into the lower respiratory tract and the lungs, which can then cause infection. They concluded that individuals with incomplete root canal treatments have a higher risk of pneumonia hospitalization.33 Another study by Lin et al. looked at unfinished root canal treatments and its relationship to cardiovascular disease, again using a nationwide population-based database. In this study there were a total of 283,590 participants who had at least one root canal, with no cardiovascular history prior to 2005. They tracked the participants until the end of 2011 and found that 3,626 patients had been hospitalized for cardiovascular disease, concluding unfinished root canals were also linked to cardiovascular disease.34
As mentioned above, microorganisms in the mouth have been linked to various diseases, including cancer, which is the second leading cause of death globally. According to the World Health Organization (WHO), lung, breast, colorectal, prostate, skin, and stomach cancer are amongst the most prevalent around the world.35 It was only in the 1990s when the first bacterial species, Helicobacter pylori, was declared as a definitive cause of cancer by the WHO. These three common microorganisms, Porphyromonas gingivalis, Tannerella forsythia (formerly Bacteroides forsythus), and Actinobacillus actinomycetemcomitans have been implicated in periodontitis.36 Research is definitively showing that there is a connection between specific microorganisms from the oral cavity and various cancers and systemic diseases; in fact, oral squamous cell carcinoma has been identified as one of the most pervasive cancers globally.37 The link between oral bacteria and its role in development of various cancers, in addition to oral cancer, are colorectal and pancreatic cancer. The most commonly identified oral bacteria related to cancer are Fusobacterium nucleatum and Porphyromonas gingivalis. Streptococcus sp., Peptostreptococcus sp., Prevotella sp., and Capnocytophaga gingivalis have also been linked in the pathology of cancer.38

Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans were associated with a higher risk of pancreatic cancer in a population-based nested case-control study from two prospective cohort studies, the American Cancer Society Cancer Prevention Study II and the National Cancer Institute Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. The significance of this study showed the specific bacteria, which can be helpful to identify high risk individuals, as well as prospective treatments to lessen the risk of pancreatic cancer.39 Other large cohort studies have been published on different types of cancer and its relationship to oral health. The connection between non-Hodgkin lymphoma and PD was explored in the Health Professionals Follow-Up Study. The original findings reported a 31% higher risk of non-Hodgkin lymphoma when individuals had severe PD at baseline. By extending the study by another eight years of follow-up, the outcome suggests that PD is a risk factor for non-Hodgkin lymphoma.40 In the Atherosclerosis Risk in Communities Study, that included 7,466 participants, six sites were measured on all teeth to identify the severity of PD. The statistical analyses were two-sided with a median of a 14.7-year follow-up. The findings showed an increased risk of total cancer for severe PD, notably for lung and colorectal cancer.41
A prospective cohort study of postmenopausal women and the relationship between PD and breast cancer was conducted by the Women’s Health Initiative Observational Study. The study followed 73,737 postmenopausal women who did not have breast cancer. The mean follow-up was 6.7 years, where 2,124 invasive breast cancer cases were found. PD was reported by 26.1% of the women, particularly among former smokers who quit within 20 years. PD was concluded to be a risk factor of postmenopausal breast cancer, especially among former smokers who quit within 20 years.42
The relationship of PD and its link to prostate cancer was investigated in a 12-year longitudinal cohort study in South Korea. The investigators used the National Health Insurance Service-Health Examinee Cohort database sample of 187,934 South Koreans, from 2002-2013. While prostate cancer is widespread in the United States and Europe, it is only the seventh leading cause of cancer death in South Korean men. However, due to the aging population in Korea, along with obesity, the rise in prostate cancer has increased dramatically. They found that this cohort study had shown that patients with PD have a significant, but slightly positive link with prostate cancer.43 The Center for Disease Control and Prevention (2012) estimate that one out of two adults over 30 in the US has PD; for adults that are over 65, the estimate is over 70 percent.44
According to Gulati et al. periopathogens can enter the bloodstream causing bacteremia. When bacteremia occurs, there are three routes where a focal infection can appear in the bloodstream and organs. They are metastatic infection from oral cavity due to bacteremia, metastatic injury due to microbial toxins, or metastatic inflammation due to immunologic injury caused by oral microorganisms.45 Both the gastrointestinal tract and the oral cavity have the most highly diversified assorted bacteria found in the human body. Due to the temperate and damp ecosystem of the oral cavity, it is an ideal habitat for microbial communities. Teeth are the only non-shedding surfaces in the human body and dental restorations such as crowns, bridgework, removable protheses and implants, also do not shed and can therefore sustain oral biofilm. The use of these biomaterials may not only have a negative effect on the oral cavity, but may also have a negative impact on a patient’s overall health and wellness.46 Biofilms develop from bacteria attaching to surfaces in the oral cavity such as the teeth, gums, or tongue. These microorganisms can be pathogenic, can cause oral infections, and may lead to dissemination and systemic disease. Dental prosthesis is prone to harbor the most invasive biofilms, more than any other medical devices. It has been found that over 65% of denture wearers suffer from stomatitis, which impacts poor oral health, dental hygiene, and systemic disease, including diabetes. Infections and inflammation can also be caused from dental implants, when bacteria accumulates in the implant area and forms a biofilm.47 The American Dental Association (2014) stated more than 5 million titanium dental implants are placed yearly in the United States and placement is expected to grow.48 It was generally thought that titanium dental implants were not only inert but also fairly biocompatible; however, research is showing that titanium dental implants can break down due to corrosion. Allergic reactions may occur from the disintegration of these implants, producing particles that migrate to other areas far from the original implant site. Traces of metal from dental implants have been found in blood, liver, lungs, and lymph nodes. Although titanium is used in dental implants, other dental uses include membranes, grids, reduction plates, and screws; and titanium is also used in root canal sealers. The International Agency for Research on Cancer has classified titanium as a possible carcinogen.49
While the majority of studies have been focusing on the gut microbiota, the actual source point is the oral cavity, which is now being investigated for its potential relationship to many diseases. According to Mitu et al., the oral microbiome is believed to be a significant cause of not only oral diseases but also many systemic diseases such as diabetes, cardiovascular diseases and various syndromes like autism spectrum disorders.50 The trillions of microorganisms, their diversity in the human body, and how they interact, require a new way of looking at the entire system, according to Le Bars et al. Changes in the oral microbiota can possibly assist in predicting the risk of cancer. This can be accomplished by using next-generation sequencing such as pyrosequencing (Roche 454) and sequencing by synthesis (Illumina), or the sequencing of the 16S rRNA gene, which has shown alterations in oral microbiota and the range and makeup between healthy patients and those with squamous cell carcinoma from the oral cavity.51
Bacteria found in root canal teeth associated with acute apical abscesses were examined using molecular methods. It was noted that this method can deliver a more accurate and consistent identification of bacteria that are difficult to identify or can’t be precisely identified by other phenotypic tests. Twenty root-canal teeth were cultured, and two hundred and twenty strains of bacteria were identified using 16S rRNA gene sequencing along with clonal analysis, demonstrating a more diverse bacterial flora than previous studies. Fifty-nine different cultivable bacteria were identified by 16S rRNA gene sequencing, belonging to six phyla, with an average number of six species per root canal. Their findings demonstrated that the microbiota of infected root canal teeth is composed mainly of anaerobic Gram-negative bacteria, with the greatest majority belonging to the phyla Firmicutes and Bacteroidetes.52
The DNA Connexions Oral Panel
The DNA Connexions Oral Panel was designed to provide patients and practitioners a comprehensive view of patient health based on the oral flora. The panel detects the presence of 88 different microbial pathogens inclusive of bacteria, viruses, fungi, and parasites. Utilizing the molecular tool of polymerase chain reaction (PCR), this direct testing method amplifies the DNA of the targeted organisms, if present.
While the 88 microbes of the Oral Panel may seem comprehensive, there are hundreds of species and millions of microbes present in the oral flora. The oral panel constituents were chosen based on the organisms present in what is considered ‘normal’ oral flora, those more commonly associated in oral disease processes, and those which are thought to potentially be involved in varying systemic diseases.
As evidenced by 1000s of DNA Connexions Oral Panels, varying microflora constituents make up the species found in the oral cavity, even with wide variation in juxtaposed locations. The oral cavity and its proximity to the bloodstream allows the facilitation of bacteremia, sepsis, and the spreading of individual species throughout the body.
Sample Collection: Oral samples are predominately collected by dental practitioners in a controlled setting. Sampling types are teeth (root canaled, nonvital), oral blood, cavitational blood, dental implants, paperpoints, tissue, bone, and Superfloss (site specific or full mouth survey). Samples are placed in sterile, single use plasticware that has been flushed with nitrogen gas to preserve the DNA during return shipping. Practitioners are instructed to freeze samples until shipping.
DNA Extraction is performed utilizing a spin column extraction protocol. Purified samples are quantified for nucleic acid purity and concentration utilizing nanodrop instrumentation.
Amplification by PCR. The Oral Panel uses ninety-six well plates containing 85 unique species-specific PCR primer sets, 5 positive controls and 1 negative control. Purified, DNA is aliquoted, and a multichannel pipette is utilized to dispense 3 ul per reaction well containing typical PCR reaction components. Plates are sealed with thermal films and amplified using Bioer Thermocyclers on a 23-step annealing gradient profile.
Gel Electrophoresis. Amplification products are run on 1.2% agarose gels, with products ranging in size from 198bp – 1020bp, visualized with ultraviolet light and ethidium bromide stain.
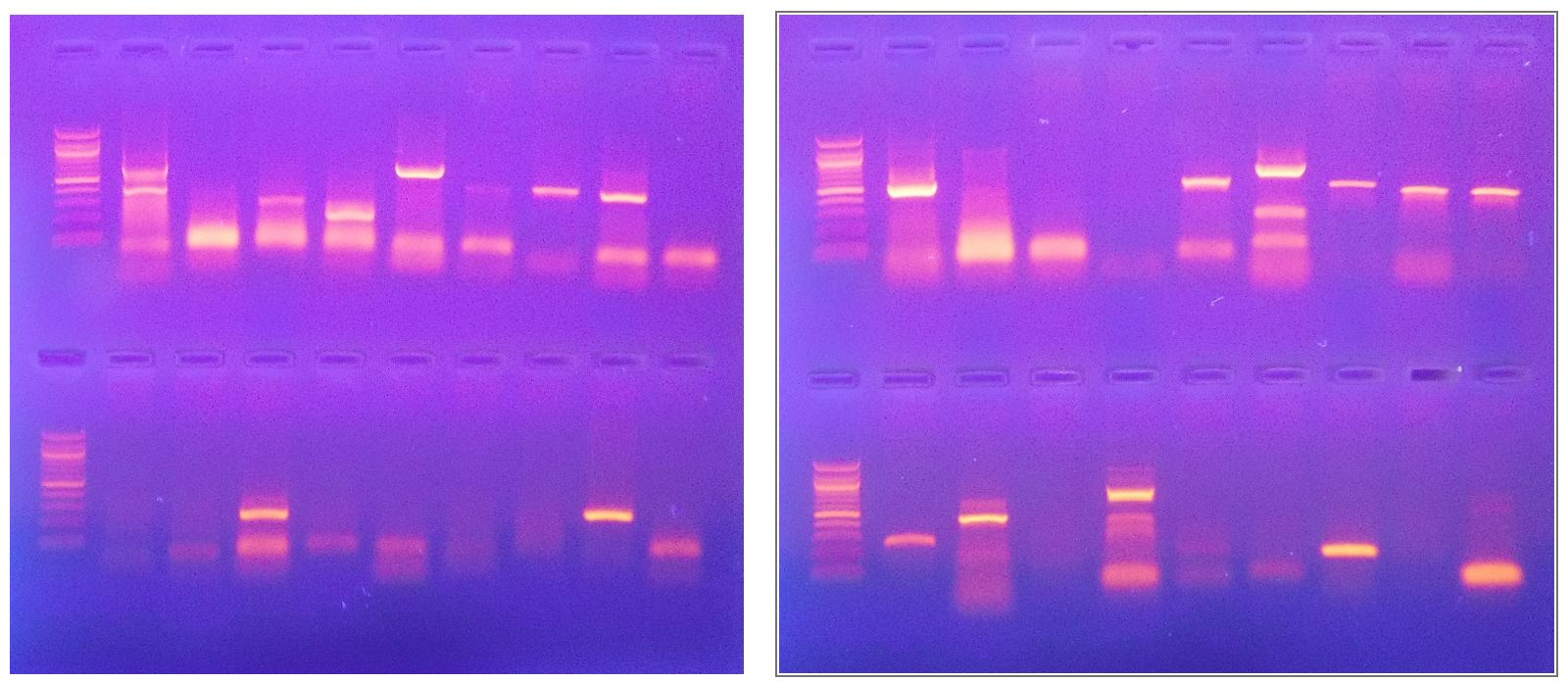
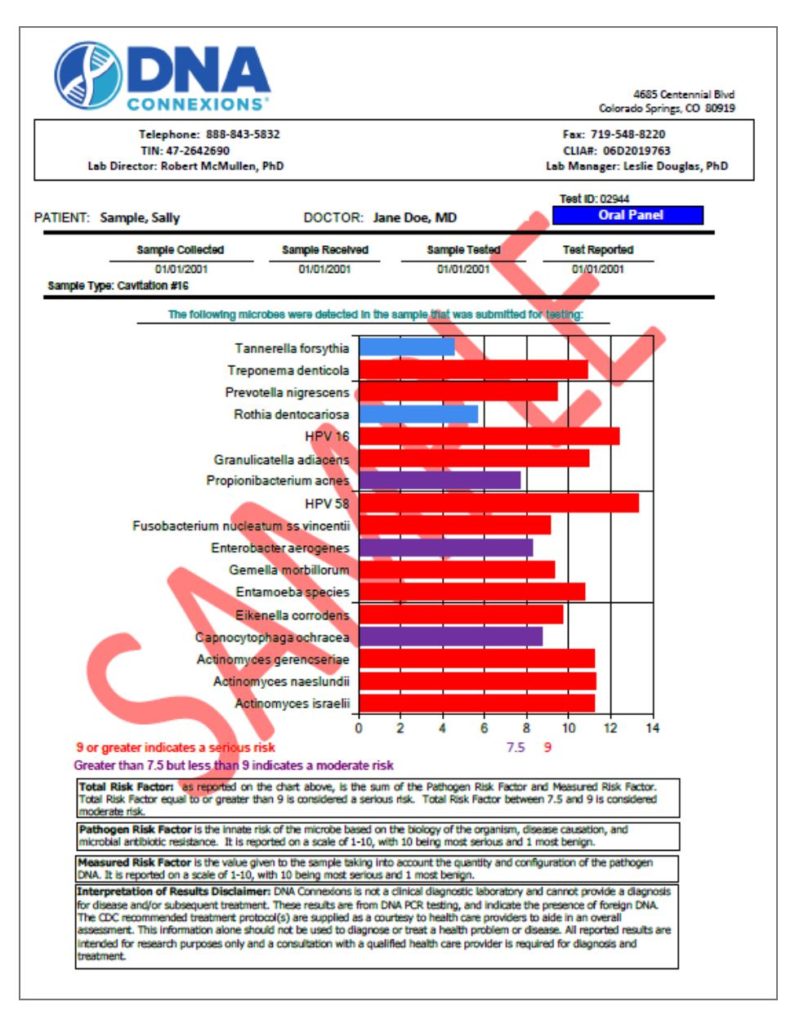
Results. The Oral Panel report provides a general description, symptoms of infection, and CDC-recommended treatment options for each organism detected.
A total risk factor is calculated for each organism, which considers the amount of the organism present (measured risk factor) and the characteristics of that organism (pathogen risk factor). Pathogen risk factors are determined based on the overall characteristics of the organism: pathogenicity, ability to cause disease, resistance to treatment, illness causing properties, links to systemic diseases, etc. The measured risk factor is based on the visualized intensity of the amplification product, which has a direct linear correlation to the amount of the organismal starting material present in the sample.
Utilizing the proprietary DNA Connexions Alpha-5 software, the combination of both the measured risk factor and the pathogen risk factor yields the total risk factor of each organism detected.
Conclusion
Poor oral health is a global pandemic, a fact that can no longer be ignored. It also cannot be overstated that there are many often-overlooked health problems that develop as a consequence of common dental procedures. Some of these universal treatments include root canal treatments, dental implants, nickel braces, or even extraction of wisdom teeth. Oral galvanism is a serious problem, especially when dissimilar metals such as mercury dental amalgam, along with titanium implants are placed in the oral cavity. Patients with existing/chronic health challenges should start by having a complete dental exam to rule out any of these treatments as the root cause of their problem. While treated teeth may appear “stable” on an x-ray and the patient may not be experiencing problems at the site, it is critical to examine these teeth utilizing the best technology that is currently available to see if infections, are in fact, present.
Many studies on the materials used in dentistry are not looking at the long-term exposure risks to the patient or the potential toxicity when these materials breakdown over time. Moreover, functionality of the restorations is often the only consideration of the dentist. As a consequence of this oversight, it is important for the doctor/dentist to study and examine the biocompatibility of each material used in all dental procedures. While there are materials that show less reactivity to many patients, there are “no one size fits all” restorative materials. The toxicity of materials is a major problem, not only in dentistry, but also in medicine. Although the toxicity of materials is being studied, few papers address the synergistic effects of exposure to different materials and more importantly, how these materials will react with the individual patient.

DNA Connexions provides testing to identify oral pathogens to assist qualified practitioners in diagnosis and treatments for their patients, including a recently added test called, the Propensity Panel, which identifies the presence of bacterial species that have been implicated in the progression of a variety of chronic and systemic conditions, as well as various cancers such as colorectal, pancreatic, prostate, and breast cancer. BIOCOMP Labs, provides biocompatibility testing so that a patient has the optimum restorative materials, specifically, to allow the least reactive restorations to be used when doing full dental revisions.
As reported in this paper, the list of diseases related to poor oral health continues to grow, particularly in the aging population. Oral pathogens are proving to be the source of many diseases, including cancer, but are rarely considered by medical professionals. This is because a colossal chasm exists between medical and dental professionals. Therefore, it is prudent not only for dentists to be alerted to the potential health risks that may be caused by common dental procedures, but also for medical doctors to look at the patient’s mouth as a potential source of infection and disease.
A simple visual examination of a patient’s mouth can often provide insight as to what may be causing health issues or can possibly prevent a potential disease risk. It is only recently that integrative medical doctors are starting to discuss the relationship of how oral health impacts whole-body health. Thus, it is also important to ensure that the dental profession is transformed from being “tooth mechanics,” to actually understanding that the foundation of whole-body health starts in the mouth, and the critical role that the dentist plays in patients’ overall health.
What we have witnessed through clinical observations over several decades is that doing a complete dental revision, utilizing the most biocompatible restorations, along with a comprehensive detox and supplementation protocol has, in fact, improved the lives of many of our patients. This is because we teach the patients to actively participate in their own health and wellness.
What can we do to really achieve extraordinary healing results for the patient instead of simply treating disease symptoms? We believe that it is finally time to create a real partnership between both the medical and dental professions and the patient, which is the only viable solution because one profession cannot do this alone; and of course, this cannot be achieved without the patient’s participation. As so eloquently spoken by Dr. Frank Billings over 100 years ago, this was worth repeating, “Anyone who has seen the illuminating results of a better physical health in the patients, whose dirty mouths have been made as nearly as possible clean, must be convinced of this source of systemic infection.”
This paper is dedicated to Dr. Hal Huggins, a man who was persecuted during his lifetime for his revolutionary and visionary work in research and in biological dentistry. Science is finally proving what he wrote so long ago was right, “It’s All in Your Head”!
Complete References are found on Page 2 –
Blanche D. Grube, graduated from Queens College, CUNY and received her doctorate from UMDNJ, now Rutgers School of Dental Medicine. She holds a second doctorate from Capital University of Integrative Medicine, Washington DC, and is a board-certified biological dentist and a past president of International Academy of Biological Dentistry and Medicine. She has lectured internationally on the Huggins-Grube Protocol. Besides holding several fellowships, she is the owner and CEO of DNA Connexions, Biocomp Laboratories, Huggins Applied Healing, and Centers for Healing.
Dr. Leslie J. Douglas completed her undergraduate studies in biology at the University of Hawaii at Hilo (UHH) before attending the University of Hawaii at Manoa (UHM), Department of Genetics and Molecular Biology. Currently, she is the Principal Investigator and Laboratory Manager of DNA Connexions, a Colorado-based company focusing on bacterial, viral, fungal, and parasitic molecular-based detection assays. Her main focus is the research and development of a PCR-based Lyme test inclusive of Borrelia burgdorferi and a number of prevalent tick-borne disease co-infections, as well as the ongoing development of various molecular-based assays. Dr. Douglas’s research and patient demographics is yielding invaluable data to better understand the relationships between Lyme and other chronic conditions.
Anita Vazquez Tibau is a researcher at the Center for Environmental and Toxicological Research at the University of Puerto Rico. She has been working on a global ban on dental mercury for two decades, working with various Non-Governmental Organizations. Since 2002, she has been an active participant in what is now called the Minamata Convention on Mercury treaty, where she wrote and delivered the closing statement on behalf of the International Academy of Oral Medicine and Toxicology. She has published articles in internationals journals and several of her articles have been translated into multiple languages. She has lectured in the US and abroad. Most recently she was the recipient of the Humanitarian Award at the Doctors Who Rock Gala, 2019, Orlando, Florida.











