By Anne Marie Fine, NMD
As the research body of evidence between the major role of toxicants and chronic disease continues to grow, the contributions of personal care products applied to and sprayed around the skin are often overlooked. There is persuasive evidence that even low-level toxic exposures cumulatively contribute to chronic diseases, making it even more likely that the single exposure model of toxicology is more the exception rather than the rule. Certain ingredients in personal care products have been found to be endocrine disruptors, neurotoxins, carcinogens, and skin sensitizers. Daily exposure to these products, even if the exposures are considered small dosages, must be considered in evaluating overall body burden of toxicants. This article seeks to provide an integrated understanding of the totality of exposome factors in human health by focusing on the contributions of personal care products via dermal absorption.
The Skin Exposome
The exposome as a concept and definition has been around for more than ten years. The exposome consists of external and internal factors and their interactions, affecting a human individual from conception to death.1 It acts on the human genome and is the environmental trigger to the expression of certain genes. However, the exposome of human skin has not received major attention both for effect on the skin itself and for effects on the body through dermal absorption. The skin plays a particularly important role in environmental exposures because it is first and foremost a barrier organ that is subjected to lifelong exposure to a large variety of environmental factors, while acting as a portal to absorb or excrete certain compounds. For this reason, I am expanding the definition of the skin exposome to encompass not only sun exposure, sleep, stress, cigarette smoking, air pollution, nutrition, but also cosmetics and personal care products.
The skin is the body’s largest organ, accounting for more than 10% of total body mass. And while its role as an organ of excretion is well-known, an opposite function, that of an organ of absorption is not at all well-known. Dermal absorption of chemicals depends on the integrity of the skin barrier function, lipophilicity of the compound, with low molecular weight chemicals with good solubility in both water and fat penetrating the skin more readily, and temperature of the skin, with higher temperature allowing more absorption. Compromised barrier function of the skin also results in increased dermal absorption of chemicals, which can lead to the entry of larger molecules such as proteins, inorganic metal compounds, or nanoparticles. Solvents are one class of chemicals that has been shown to reduce barrier function of the skin by damaging lipid and protein structures of the stratum corneum. Many personal care product formulations contain dermal penetration enhancers such as alcohol and propylene glycol, which also carry certain ingredients deeper into the dermis. Medicines, including topically applied hormones or nicotine aids, can also be driven into the skin dermally through patches or creams, which typically contain a penetration enhancer.
The ability of dermally applied ingredients to penetrate the skin and reach the circulatory system has been established. Certain ingredients from personal care products such as parabens, phthalates, and BPA have been found not only in maternal serum, but in cord blood of newborns, amniotic fluid, and breastmilk.2 We can no longer pretend that the skin is an impenetrable barrier. However, the internet and some self-proclaimed “experts” commonly promote the idea that 60% of what is applied to the skin is absorbed into the bloodstream. This derives from a single study in 1984,3 which demonstrated that an average of 60% of solvents from drinking water were absorbed dermally via bathing. However, this study cannot be extrapolated to apply to all substances applied to the skin, because as previously discussed, differences in molecular composition and solubility affect absorption.
In one study of healthy men, it was demonstrated that after applying a body lotion containing 2% each butylparaben, diethyl phthalate (DEP), and dibutyl phthalate (DBP), the butyl paraben was detectable in the serum after only one hour, and in urine with a peak value 8- 12 hours later.4 The main phthalate metabolites, monoethyl (MEP) and monobutyl phthalate (MBP), were found in the serum as well.
Bisphenol A (BPA) is another chemical compound that easily and quickly enters the systemic circulation. While it is felt that BPA exposure is largely due to oral ingestion from consuming food or water stored in plastic containers or cans lined with BPA, studies have also demonstrated dermal absorption through thermal receipts that are coated in free BPA.5
More recent evidence now points to dermal absorption through ambient air exposure as a meaningful exposure pathway for phthalates, in addition to the expected mechanisms of oral absorption and dermal application.6
Due to the short half-lives of some of these chemicals found in personal care products, simply removing them and substituting products that don’t contain the problematic substances has shown good results in reduction of urinary metabolites in remarkably short periods of time. For example, the Hermosa study was an interventional study of adolescent girls that demonstrated significant reductions in urinary levels of parabens, triclosan, and phthalates, over a three-day period of substituting personal care products that did not contain those chemicals.7
Endocrine Disruptors
Endocrine disrupting chemicals (EDCs) alter hormonal signaling, affect developing reproductive systems, and disrupt mammary development.8 Often the impact of endocrine disruptors is highest at times of greatest developmental growth, such as in utero, puberty, and pregnancy. Epidemiological data show increases in incidence and prevalence of diseases associated with endocrine disrupting chemicals such as breast, prostate and testicular cancers, diabetes, obesity, and decreased fertility over the last 50 years.9 Endocrine disruptors in personal care products include parabens, phthalates, and triclosan.
Paraben esters act as estrogen agonists, androgen antagonists, inhibitors of sulfotransferase enzymes, and have genotoxic activity.10 Recently, parabens were shown to impair human sperm motility, enhance the generation of mitochondrial ROS, and stimulate the formation of oxidative DNA adducts.11 While parabens have never been formally classified as a carcinogen, they have been shown to enable multiple hallmarks of cancer, including resistance to cell death, sustained proliferation, evasion of growth suppressors, invasion and metastasis, genome instability, and deregulation of energy metabolism. 12 Parabens have been found in 99% of breast tissue samples and can stimulate sustained proliferation of human breast cancer cells at concentrations measurable in the breast.13
Phthalates are a group of chemical compounds consisting of high molecular weight phthalates and low molecular weight phthalates. The low molecular weight phthalates are found in personal care products as dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), and diisobutyl phthalate (DIBP). High molecular weight phthalates such as di(ethylhexyl) phthalate (DEHP) are a ubiquitous type of plasticizers used in a wide range of consumer products including toys, food packaging, cosmetic products, and medical equipment. DEHP represents a particular public health concern because 100% of the US population have measurable levels of this endocrine disrupting compound. Phthalates have been found to be associated with significantly earlier menopause in women, decreased couple fecundity, low birth weight, preterm birth, altered placental methylome and transcriptome, and pregnancy loss.14
In men, phthalates have been associated with altered semen parameters as noted in Table 1, below.15 In addition to potential contributions to the deterioration of sperm quality, phthalates have also been found to be associated with anatomical abnormalities in male fetuses such as shorter anogenital distances, a marker for potential reproductive problems.16,17
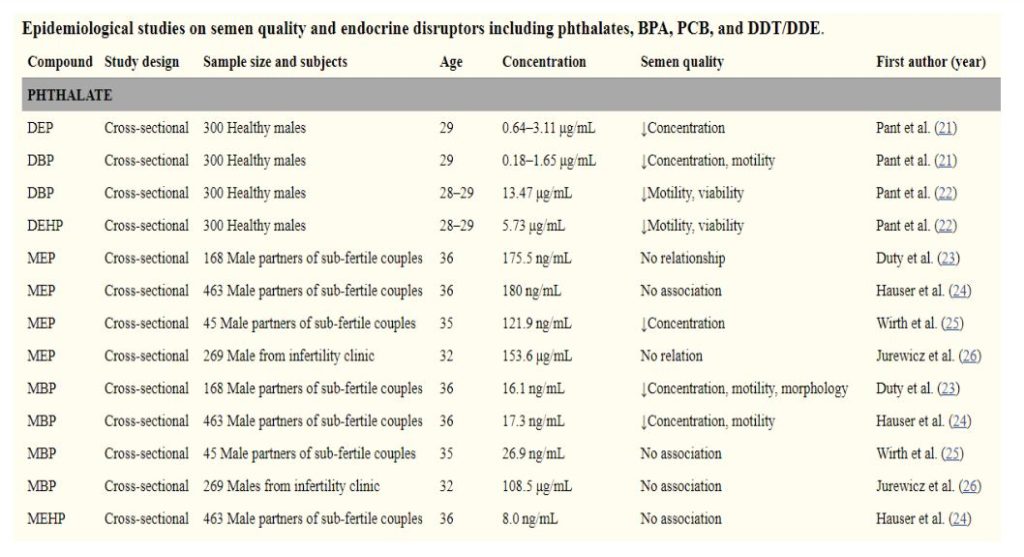
Phthalates also join other environmental exposures that increase the odds of diabetes. Women who were in the third and fourth quartile of phthalates had nearly twice the odds ratio of developing diabetes than women in the first quartile.18 Phthalates during pregnancy also were associated with a higher risk for higher weight gain and glucose intolerance, which are both risk
factors for gestational diabetes.19
While phthalate exposure through ingestion, inhalation, and dermal contact are considered important routes of exposure for the general population, recent research has uncovered a new exposure: ambient air.20 This adds another layer of complexity upon the concept of avoidance, as even if a person scrupulously avoids the use of phthalates from cosmetics and plastics, simply being in the vicinity of other phthalate emitters puts that person at risk. This has been borne out by a recent study that examined 23 cosmetics, four perfume, and nine clothing department clerks for changes in urinary phthalates pre and post-shift. Post-shift urinary levels of mono-2-ethylhexyl phthalate (MEHP- a metabolite of DEHP) and monomethyl phthalate (MMP-a metabolite of DMP) were significantly higher for the cosmetics and perfume clerks than for the clothing clerks. Median levels of air diethyl phthalate (DEP) in cosmetics (1.77 μg/m 3 ) and perfume (1.75 μg/m 3 ) groups and di-(2-ethylhexyl) phthalate (DEHP) in perfume group (6.98 μg/m 3 ) were higher than those in clothing group (DEP: 0.89; DEHP: 2.16 μg/m 3 ). Over half of cosmetic (70%) and perfume sale clerks had exceeded cumulative risk of phthalate exposure for anti-androgenic effect. The authors concluded that cosmetic and perfume workers had increased risks of reproductive or hepatic effects for DBP and DEHP exposure and suggest that not only inhalation but dermal exposure through ambient air is important route of phthalate exposure for cosmetics and perfume workers.21
Originally registered as a pesticide by the EPA, triclosan is a chlorinated aromatic compound that has been used as an antimicrobial agent since the 1970s. It has widespread use in consumer goods like anti-bacterial soaps, kitchen utensils, toys, bedding, and clothing, making it easy to see how 75% of people tested have been exposed.22 In personal care products, it enjoyed a near monopoly on the antimicrobial soaps for home use that came into fashion in the 1990s. The FDA banned triclosan for use in antimicrobial hand soaps in 2016 due to concern about its estrogenic and androgenic activities in vitro, and adverse effects reported on reproductive function in rodents and aquatic species, as well as not being found to be any more effective in neutralizing bacteria than plain soap and water. However, triclosan can still be found in other consumer products such as toothpaste. In more recent findings, triclosan is now being found in indoor air, with concentrations varying according to location; office space was the most contaminated, followed by apartment space, house space, and day nursery space.23
Neurotoxins
Lead and aluminum are the primary neurotoxins found in personal care products. The FDA
conducted a study that found lead in 100% of all the lipsticks, with tested levels up to 7.19 ppm.24
FDA Analyses of Lead in Lipsticks – Expanded Survey
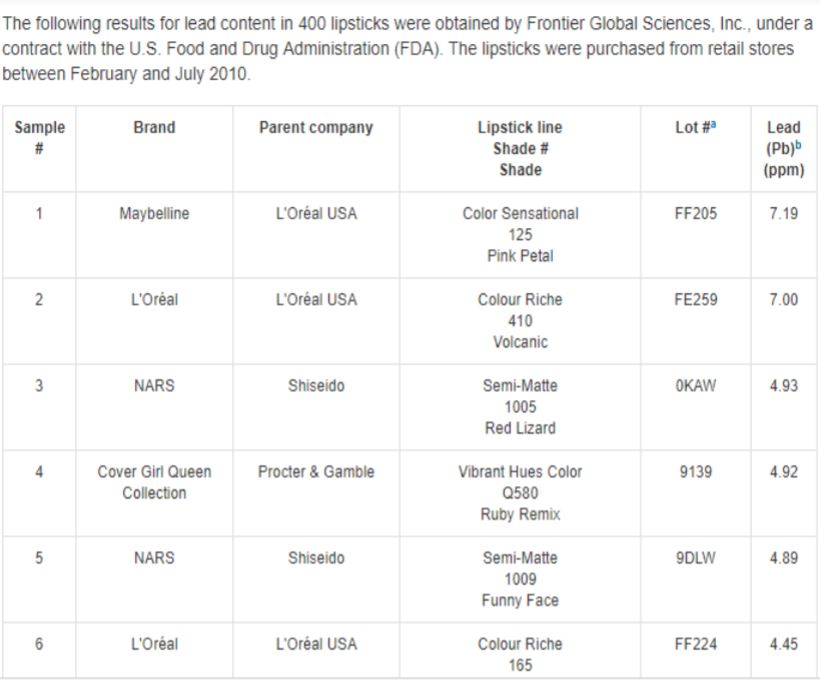
Lead from lipstick poses harm because exposure is due to ingestion; after all, lipstick commonly winds up on teeth or the food that the woman is eating, which is why lipstick is commonly applied several times per day. As no level of lead has been proven to be safe in children according to the CDC, pregnant and breastfeeding women are a particularly vulnerable population.25 The CDC reports that even low levels of lead in children’s blood have been shown to affect IQ, attention span, and academic achievement.
Aluminum is widely found in anti-perspirants and is considered a neurotoxin and metalloestrogen. It has been found in breast tissue and fluids at higher levels than in blood. Over time, breast cancer incidence in the upper outer quadrant of the breast has increased, leading to questions of whether or not aluminum-containing underarm cosmetics may be contributing to this phenomenon.26 In one small study analyzing nipple aspirate from women with and without cancer, significantly increased aluminum and pro-inflammatory and chemoattractant cytokines were found in the women with cancer, suggesting that aluminum accumulation in the breast may be a possible risk factor for breast cancer.27 In a recent case control study, the use of underarm cosmetic products containing aluminum salts multiple times per day commencing prior to age 30 had a significant increase in breast cancer risk (OR of 3.88 (95% CI 1.03–14.66)).28
Carcinogens
Several agents listed by the IARC as Group 1 carcinogens can be found in personal care products: formaldehyde, 1-4 dioxane, and ethylene oxide.29 For the most part, these are found as contaminants in formulations except for formaldehyde, which is either intentionally added to formulations such as certain hair-straightening products, or released slowly into a product from a formaldehyde-releasing preservative system. Because of this fact, they will not be explicitly listed on a label as “formaldehyde,” making it important to know how to determine its presence.
In the case of formaldehyde-releasing preservative systems, the chemicals that are listed on the label slowly release formaldehyde over time, which preserves the product. The health concerns with formaldehyde releasers include direct toxic effects and contact dermatitis for many users. In fact, the American Contact Dermatitis Society awarded formaldehyde its dubious honor of being the 2015 contact allergen of the year. Twenty of all cosmetic products, including 17% of stay-on products and 27% of wash-off products contain formaldehyde releasers. These formaldehyde-releasing preservative system chemicals include the following:
Quaternium-15,
Dimethyl-dimethyl (DMDM),
Hydantoin,
Imidazolidinyl urea,
Diazolidinyl urea,
2-bromo-2-nitropropane-1,3-diol (bronopol),
Sodium hydroxylmethylglycinate.30
Both 1-4 dioxane and ethylene oxide are by-products of the ethoxylation process, which is a method used to make certain agents less harsh by reacting them with ethylene oxide. This process can result in small amounts of 1-4 dioxane and residual ethylene oxide in the product. You will not know if this appears in the product of question because these are an unintentional byproduct of the ethoxylation process and may or may not be present at all since some companies use vacuum stripping to remove them. However, if the label contains the following chemicals that have been subject to the ethoxylation process, then the contaminants might appear in the final product:
Sodium laureth sulfate,
Polyethylene,
Polyethylene glycol (PEG),
Polyoxyethylene,
Polysorbate,
Or any ingredient with “xynol,” “ceteareth,” or “oleth.”30
Conclusion
When assessing the entirety of the exposome that affects human health, it is important to consider the invisible sea of synthetic chemicals being applied to our patients’ bodies every day. Consumer awareness about human health concerns associated with various ingredients used in personal care products is increasing markedly. This has placed considerable demand on the companies that formulate these products to produce safer products even in the face of regulations that have simply not kept up with the science. Indeed, other countries are already adhering to the Precautionary Principle and formulating safer products for their people through the lens of stricter regulations. As we evaluate our patients’ toxic body burden with appropriate attention paid to the daily use of myriad personal care products, we can identify problematic exposures and make well-informed suggestions for replacement products.
Anne Marie Fine, NMD, is a practicing naturopathic doctor focused on environmental medicine who is based in Newport Beach, California.
She currently serves as vice-president for the National Association of Environmental Medicine, teaching healthcare providers this emerging specialty. She is also a member of the American Academy of Environmental Medicine and a science advisor for the non-profit MadeSafe.org.

Fine speaks and consults globally within the personal care product industry and guides companies to formulate with cleaner ingredients.
Dr. Fine has been published in peer-reviewed medical journals, Thrive Global, and popular magazines alike. She is featured regularly on health podcasts, TV, and radio shows. Dr. Fine is also the international bestselling author of Cracking the Beauty Code: How to program your DNA for health, vitality, and younger-looking skin, a book that synthesizes her knowledge of skin aging, gene-environment interactions, and environmental medicine. She is also the founder and CEO of IAMFINE®, an integrative beauty and wellness brand that empowers women to take control of their health and beauty.
For more information see www.drannemariefine.com.
References
1. Krutmann J, et al. The Skin Aging Exposome. Journal of Dermatological Science. 2017 March;85(3):152-161.
2. Mitro SD, Johnson T, Zota AR. Cumulative Chemical Exposures During Pregnancy and Early Development. Current Environmental Health Reports. 2015;2(4):367-378..
3. Brown HS, Bishop DR, Rowan CA. The role of skin absorption as a route of exposure for volatile organic compounds (VOCs) in drinking water. American Journal of Public Health. 1984;74(5):479-484.
4. Janjua NR, et al. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ Sci Technol. 2007 Aug 1;41(15):5564-70.
5. Ehrlich S, et al. Handling of Thermal Receipts as a Source of Exposure to Bisphenol A. JAMA. 2014;311(8):859-860.
6. Weschler CJ, et al. Transdermal uptake of diethyl phthalate and Di(n-butyl) phthalate directly from air: Experimental verification. Environ Health Perspect. 2015 Oct;123(10):928-34.
7. Harley K, et al. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ Health Perspect. 2016 Oct;124(10):1600-1607.
8. Taylor KW, et al. Associations between Personal Care Product Use Patterns and Breast Cancer Risk among White and Black women in the Sister Study. Environ Health Perspect. 2018 Feb 21;126(2): 027011.
9. DeCoster S, van Larebeke N. Endocrine disrupting chemicals: Associated disorders and Mechanisms of Action. Journal of Environmental and Public Health. 2012; Article ID 713696
10. Darbre PD, Harvey PW. Paraben Esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008 July;28(5):561-578.
11. Samarasinghe SVAC, et al. Parabens generate reactive oxygen species in human spermatozoa. Andrology. 2018 May 2.
12. Darbre PD, Harvey PW. Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: a review of the literature with reference to exposure data and regulatory status. J ApplToxicol. 2014 Sept;34(9):925-38.
13. Barr L, et al Measurement ofparaben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012 Mar;32(3):219-32.
14. Grindler NM, et al. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Scientific Reports. 2018;8:6086.
15. Jeng HA. Exposure to Endocrine Disrupting Chemicals and Male Reproductive Health. Frontiers in Public Health. 2014;2:55.
16. Bornehag C-G, et al. Prenatal Phthalate Exposures and Anogenital Distance in Swedish Boys. Environmental Health Perspectives. 2015;123(1):101-107.
17. Swan SH, et al. First trimester phthalate exposure and anogenital distance in newborns. Human Reproduction (Oxford, England). 2015;30(4):963-972.
18. James-Todd T, et al. Urinary Phthalate Metabolite Concentrations and Diabetes among Women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environmental Health Perspectives. 2012;120(9):1307-1313.
19. James-Todd T, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment international. 2016;96:118-126.
20. Weschler CJ, et al. Transdermal Uptake of Diethyl Phthalate and Di(n-butyl) Phthalate Directly from Air: Experimental Verification. Environmental Health Perspectives. 2015;123(10):928-934.
21. Huang PC, et al. Characterization of phthalates exposure and risk for cosmetics and perfume sales clerks. Environ Pollut. 2018 Feb;23”577-587.
22. Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Re 2017;20(8):447-460.
23. Darbre PD. Overview of air pollution and endocrine disorders. International Journal of General Medicine. 2018;11:191-207.
24. https://www.fda.gov/cosmetics/productsingredients/products/ucm137224.htm#expanded_survey.
Accessed 6/29/18.
25. https://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm. Accessed 7/20/18.
26. Darbre PD. Aluminum and the human breast. Morphology 2016;100(329):65-74.
27. Mannello F, Ligi D, Canale M. Aluminum, carbonyls and cytokines in human nipple aspirate fluids: possible relationship between inflammation, oxidative stress, and breast cancer microenvironment. J Inorg Biochem. 2013 November:128:250-256.
28. Linhart C, et al. Use of Underarm Cosmetic Products in Relation to Risk of Breast Cancer: A Case-Control Study. EBioMedicine. 2017;21:79-85.
29. http://www.safecosmetics.org/get-the-facts/chemicals-of-concern/known-carcinogens/. Accessed 7/2/18.
30. Fine AM. Cracking the Beauty Code: How to program your DNA for health, vitality, and younger-looking skin. Newport Beach, CA; Fine Natural Products: 2017.










