|
Part 1, Part 3 and Part 4 are also online.
Studies Suggesting Efficacy of Nutritional Cancer Supplementation
Small Cell Lung Cancer Study by Jaakkola
There aren't many studies evaluating the efficacy of nutritional supplements, in part because there just isn't the economic motivation to do these studies, since supplements are not patentable and the vast majority of clinical research is carried out by pharmaceutical companies on clinical trials for patentable drugs. Nevertheless, there are a few suggestive studies, but most physicians aren't aware of them. Even fewer studies have been done on a combination of a variety of supplements. One nonrandomized study carried out in Finland and published in 1992 involved 18 patients with small cell lung cancer where patients received a number of vitamins and minerals (several in relatively high doses), along with conventional treatment.25 The vitamin supplements with dosages used in the study are found in Table 1 and a list of minerals used is found in Table 2.
Table 1: Dosages of Vitamins, Minerals and Essential Fatty Acids – Jaakkola Study
Retinol palmitate (vitamin A)........................ 15,000 to 40,000 IU
Beta carotene.............................................. 10,000 to 20,000 IU
Alpha tocopherol acetate (vitamin E) ........... 300 to 800 IU
Thiamin hydrochloride (vitamin B1) ............. 150 to 750 mg
Pyridoxine HCI (vitamin B6) ....................... 200 to 1140 mg
Cyanocobalamin (vitamin B12) ................... 30 to 1600 mcg
Nicotinamide (vitamin B3) .......................... 150 to 400 mg
Vitamin D................................................... 400 to 1000 IU
Ascorbic acid (vitamin C) ........................... 2000 to 5000 mg
Calcium pantothenate (vitamin B5) .............. 50 to 300 mg
Biotin ......................................................... 300 to 1000 mcg
Essential fatty acids ..................................... 5 to 65 g
Table 2: Minerals Used in Jaakkola Study
Calcium Magnesium
Zinc Manganese
Selenium Copper
Chromium Vanadium
The endpoint for the study was a simple one, namely the survival time of the patients from the time of diagnosis compared with the survival statistics of the US National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program for a similar group of patients. The survival statistics are shown in Figure 2. From the time of diagnosis, at 6 months, the survival of the SEER group was 50% and the Nutrient group almost 95%; at 12 months, 20% of the SEER group and 85% of the Nutrient group was still alive; at 24 months, survival for the SEER group was only 10%, while 55% of the Nutrient group was still alive; at 30 months, only about 1% of the SEER group was still alive while 40% of the Nutrient group was still living, and finally at 6 years or 72 months, all of the SEER group had passed on, while 44% (8 of 18 patients) of the Nutrient group was still alive.
Figure 2
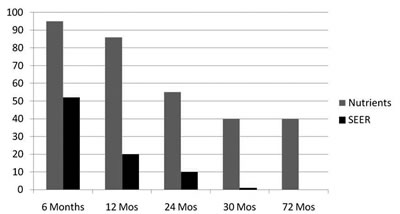
Conclusions of the study were: (1) Antioxidants and other nutrients given to small-cell lung cancer patients along with conventional treatment drastically improved long-term survival; (2) "(There) were no side effects observed (from nutrients)"; (3) "Surviving patients started AOX treatment earlier than those who succumbed"; (4) "AOX treatment should start as early as possible in combination with chemo &/or radiation." Granted, this was a very small study, but the statistics are truly amazing. An unbiased observer would expect that this study would have at least provoked some interest and an attempt would have been made to replicate it, but I could find no evidence of this in the medical literature.
Studies of Abram Hoffer, MD, on Advanced Cancer Patients Using High Doses of Nutritional Supplements Preceded by Studies of Cameron and Pauling Using High Doses of Vitamin C (Ascorbate): Conflicts with the Mayo Clinic Studies
Linus Pauling, two-time Nobel Prize winner, was first introduced to the concept of high-dose vitamin C by biochemist Irwin Stone in 1966. Being convinced of its worth and championing its use for the common cold, Pauling began to collaborate with Scottish cancer surgeon Ewan Cameron in 1971 on the use of intravenous and oral vitamin C as cancer therapy for terminal cancer patients. The reasoning was that cancer patients were generally depleted of ascorbate, and ascorbate had numerous anticancer activities. They conducted a study involving 100 terminal cancer patients in a Scottish hospital. After 10 days of intravenous vitamin C therapy, each patient was given 10 grams of vitamin C orally each day indefinitely. Their progress was compared with that of 1000 similar patients treated identically, but who received no supplemental ascorbate. The mean survival time for the ascorbate group was 4.2 times more than the control subjects (more than 210 days compared with 50 days for the controls). An analysis of the survival-time curves indicated that deaths occurred for about 90% of the ascorbate-treated patients at one-third the rate for the controls and that the other 10% had a much greater survival time, averaging more than 20 times the controls.26,27 Cameron and Pauling concluded that high doses of vitamin C should be given to all cancer patients.
The medical establishment rejected the conclusions of Cameron and Pauling after a series of papers from the Mayo Clinic failed to confirm their findings.28,29 Pauling bitterly criticized these studies and claimed that they did not replicate his studies.30 For one thing, in the first study, it turned out that the vast majority of cancer patients in the Scottish study were hospitalized and could be followed very closely and very few of them had previously received chemotherapy (only 4%, though the Moertel published study erroneously indicated that 50% had received chemotherapy), whereas virtually all of the cancer patients in the Mayo Clinic study had received previous chemotherapy.
In the second study, the Mayo Clinic patients (colon cancer patients with metastases for which there was no known effective conventional treatment) had not received any chemotherapy, but again Cameron and Pauling were critical of this Moertel study. First of all, very few of the control patients were checked to see if they were taking vitamin C on their own. This could be done by checking their urines. When the urine of a few patients in the control group were checked, it turned out that one or more of them were taking C on their own, making the control group invalid. Another major difference between the Moertel study and the original Cameron and Pauling study was that patients in the Cameron-Pauling study were given 10 grams of vitamin C until they died, whereas the Mayo Clinic patients were given vitamin C until they showed progression of their disease (increase in size of the tumor), at which point they were abruptly stopped from taking ascorbate and given other conventional treatments instead. Cameron and Pauling had not claimed that vitamin C cured cancer or even that it caused shrinkage of cancerous tumors. They claimed that it slowed the progression of the disease, increased survival time when taken consistently, and improved quality of life. This was demonstrated by comparing survival times with 1000 patients who were carefully matched as historical controls.
Moertel's experimental design did not address these issues. Instead, they treated ascorbate as a cytotoxic drug and measured its effectiveness by determining that it didn't shrink any tumors. It was then declared useless. After completion of the Mayo Clinic studies, conventional medicine concluded that vitamin C was useless for cancer patients, in spite of letters from Pauling and Cameron criticizing the experimental design and the conclusions. Vitamin C was relegated to use only by "alternative practitioners."
One recent study outlined the possible proposed mechanisms for ascorbic acid activity in the prevention and treatment of cancer.31 They are:
• enhancement of the immune system by increased lymphocyte production and activity;
• stimulation of collagen formation, necessary for "walling off" tumors;
• inhibition of hyaluronidase by keeping the ground substance around the tumor intact and preventing metastasis;
• inhibition of oncogenic viruses;
• correction of ascorbate deficiency commonly seen in cancer patients;
• expedition of wound healing after cancer surgery;
• enhancement of the anticarcinogenic effect of certain chemotherapy drugs;
• reduction of the toxicity of chemotherapeutic agents;
• prevention of cellular free radical damage;
• production of hydrogen peroxide; and
• neutralization of carcinogenic substances.
Previously, Cameron and/or Pauling had published several papers, outlining some of these mechanisms.32-34
Recent studies from the National Institutes of Health (NIH) suggest that high doses of vitamin C (achieved with intravenous doses of ascorbate) induce cancer cell death without harming normal cells. Although these studies have awakened some interest in vitamin C for cancer patients,35 most cancer specialists today still regard vitamin C as either having no effect or being harmful to cancer patients. Furthermore, although these studies were done at the NIH, the National Cancer Institute (NCI) has not shown any interest in pursuing this line of research.
In the early 1980s, Abram Hoffer MD (who did the first randomized, double-blind studies in psychiatry, using high doses of niacin for schizophrenia in the mid-1950s), evaluated a schizophrenic patient for treatment with high doses of niacin and vitamin C. This woman also had a lymphoma. Not only did the patient recover from schizophrenia, but much to the surprise of Hoffer, her lymphoma also went into remission. The word got out and soon Hoffer was bombarded with requests from cancer patients to be put on a nutritional regimen. At the urging of Dr. Pauling, Hoffer began to keep track of all of the cancer patients whom he put on this nutritional program and reported on the survival time of these patients in a series of articles.
The patients followed by Hoffer had received conventional treatments and 90% of them were considered to be advanced. The endpoint of the study for each patient was either death or survival time at the time of the inquiry. Time was measured from the first visit with Hoffer. The control group consisted of patients who approached Hoffer, but did not remain in the program for at least 2 months. Excluded were all patients who died during the first 2 months, whether they planned to continue the program or had decided not to do so.36 This study and related studies are available online at: http://orthomolecular.org/library/jom. Click on Search after inserting the search words cancer, Hoffer.
The Hoffer protocol given to the treated patients included:
(1) improved diet with the elimination of so-called junk foods (refined, processed foods containing sugar, white flour and additives); low fat and elimination of allergic foods;
(2) vitamin C 10 to 40 grams a day by mouth;
(3) vitamin B3 (niacin or niacinamide) 300 mg to 3,000 mg daily;
(4) vitamin B6 200 to 300 mg daily;
(5) folic acid 1 to 30 mg daily;
(6) vitamin E succinate 400 to 1200 IU daily;
(7) mixed carotenoids, as carrot juice;
(7) multivitamin and mineral;
(8) coenzyme Q10 300 mg to 600 mg daily; selenium 200 to 1000 mcg daily;
(9) zinc 25 to 100 mg (with some copper);
(10) calcium and magnesium in a 2:1 ratio.
Most nutrients were given in divided dosages two to three times daily.
The survival statistics for Hoffer's first 131 patients treated between 1976 and 1988 are shown in Table 3. At the end of 1 year, 28% of the controls were alive compared with 77% of the treated group. At 3 years, 16% of the control group was alive compared with 56% of the treated group. By 5 years, 5% of the control group and 46% of the treated group were alive, while at 7 and 9 years, there were no survivors in the control group, but 39% and 34% respectively in the treated group. The survival statistics for 769 patients through 1997 are shown in Table 4. Again, we see a marked difference in survival each year up until 5 years.
The conclusions from the Hoffer studies were: (1) patients with a wide variety of advanced cancers have significantly improved survival when a nutritional program is added to their conventional treatment; (2) the nutritional program consisted of dietary suggestions and relatively high doses of vitamins, minerals, and other nutritional supplements.
Intravenous Vitamin C for Cancer Patients
The first recommendations for IV vitamin C for cancer patients appeared in 1971.37 In their book on cancer and vitamin C, Cameron and Pauling summarized their work with vitamin C for cancer patients both orally and by intravenous use.38 In their study, IV vitamin C at 10 grams was administered daily for 10 days. In 1990,the late Hugh Riordan, MD, and his group in Wichita (Kansas) reported a rather amazing case study of a patient with kidney cancer who had a long-term remission with IV treatments of vitamin C in the range of about 15 to 30 grams, a few times a week.39 A paper in 1995 by Riordan's group described IV ascorbate as a tumor cytotoxic chemotherapeutic agent.40 They reported that ascorbic acid and its salts are preferentially toxic to tumor cells in vitro and in vivo and that "given in high enough doses to maintain plasma concentrations above levels that have been shown to be toxic to tumor cells in vitro, ascorbic acid has the potential to selectively kill cancer cells in a manner similar to other tumor cytotoxic agents." A major point here is that at these concentrations, ascorbic acid is not toxic to normal cells.
Table 3: Hoffer's First 131 Cancer Patients Treated from 1976 to 1988
Group Treated Untreated
Total Number 97 18
Alive at 1 year 77% 28%
Alive at 3 years 56% 16%
Alive at 5 years 46% 5%
Alive at 7 years 39% 0%
Alive at 9 years 34% 0%
Table 4: Hoffer's Cancer Patients Seen before the End of 1997
(71 Excluded)
Group Treated Untreated
Total Number 769 75
Alive at 1 year 72% 24%
Alive at 2 years 48% 12%
Alive at 3 years 37% 12%
Alive at 4 years 30% 8%
Alive at 5 years 23% 8%
Mark Levine, MD, at the NIH wrote a commentary in the Journal of the American College of Nutrition in 2000 pointing out that concentrations in the bloodstream of IV vitamin C were capable of killing cancer cells and not normal cells and that "ascorbate treatment of cancer should be reexamined by rigorous scientific scrutiny in the light of new evidence."41 As mentioned previously, Levine and his group at the NIH published an extremely important paper in 2005, showing that high concentrations of ascorbate (achievable by IV infusions, but not by oral doses) were capable of killing a wide range of cancer cells without harming normal cells. Furthermore, he described the mechanism by which this occurs. Ascorbate in these high concentrations acted as a pro-drug, forming hydrogen peroxide in the extracellular spaces. It is the hydrogen peroxide that is capable of killing many cancer cells and not normal cells at these concentrations.26 The reason for the discrepancy in ascorbate's ability to kill cancer cells and not normal cells may be that cancer cells have between 10 and 100 times less catalase than normal cells.30,42 Catalase is the enzyme that breaks down hydrogen peroxide in the body and with less catalase, cancer cells might be expected to be more easily killed by hydrogen peroxide. More case studies were published in 2006.43 In this paper, the authors describe: "3 well-documented cases of advanced cancers, confirmed by histopathologic review, where patient had unexpectedly long survival times after receiving high-dose intravenous vitamin C therapy." They suggested that the role of high-dose intravenous vitamin C therapy in cancer treatment should be reassessed. A nice review of ascorbic acid for cancer over the previous 25 years appeared in 2005.44
Recently, John Hoffer, MD (son of Abram Hoffer), and a professor at McGill University reported at a meeting in Orthomolecular Medicine (2009) that a clinical trial that he had run on advanced cancer patients using high-dose IV ascorbate over a 6-month period, failed to show any objective changes in the size of the tumor. The conclusion was that the IV ascorbate alone was not effective for the treatment of advanced cancers. However, J. Hoffer used the same methods that are used to evaluate toxic chemotherapeutic drugs and similar to the methods used by Moertel in his studies described previously. Measuring the size of a tumor does not necessarily correlate well with either survival time or quality of life. So, it is possible that a longer-term study that looked at the issues of survival time and various lifestyle parameters might show a different story. But it would undoubtedly be difficult to get funding for such a study. On the other hand, a study involving IV ascorbate along with a chemotherapeutic agent would be funded more easily. Currently, J. Hoffer is recruiting for a clinical trial using high-dose vitamin C with chemotherapy. This is similar to a trial run at the University of Kansas by Dr. Jeanne Drisko, which has not yet been published. It is not clear at this time if clinical trials of this sort using IV vitamin C along with chemotherapy will show vitamin C to be of benefit, but I suspect that if parameters such as survival and quality of life are measured, we should expect positive results. In my clinical experience, patients undergoing chemotherapy with another physician, but receiving high-dose intravenous ascorbate at our office in between their chemotherapy treatments, invariably report that they appear to be doing better than other patients at the oncologist's office who are not receiving high dose vitamin C.
In our practice at the Schachter Center for Complementary Medicine in Suffern, New York (www.mbschachter.com), we have been using high-dose IV ascorbate (10 to 120 gram infusions) in cancer patients for more than 30 years. Each patient receives a comprehensive program involving dietary suggestions, a variety of nutritional supplements, an exercise program, stress management program, and other lifestyle-enhancing suggestions. Our patients appear to do very well, and we believe that the C infusions play an important role in their treatment. We usually give about 60 grams of vitamin C, 10 cc of calcium gluconate, and 4 cc of magnesium chloride in 500 cc of sterile water and administer this over about 2 hours.
Historical Background of Amygdalin (Also known as Laetrile and Vitamin B17)
Historical Background
Amygdalin has been one of the most controversial cancer treatments for the past 50 years. The story involves not only science, health care, and treatment for cancer, but also politics and economics. In the US, during the 1970s and 1980s, a great debate waged between conventional medicine (with the support of the federal government) on the one hand and patients treated successfully with it, a small group of scientists and practitioners who believed in its value, and a conservative political group that fostered the notion of freedom of choice in health care, on the other. As many as 20 US states passed state legislation that decriminalized it, while some doctors who used it lost their medical licenses and some even wound up in prison. My first exposure to alternative cancer therapies was a narrated film strip titled World without Cancer, which is available on the Internet at: http://video.google.com/videoplay?docid=4312930190281243507. It is also available as a book: World without Cancer, by Edward Griffin.45 The thesis of this book, based on the theory of Ernest Krebs Jr., is that cancer is largely a nutritional deficiency disease, much like pellagra (vitamin B3), scurvy (vitamin C), or beriberi (vitamin B1) and that modern civilization ingests very little of this vitamin, which is contained in nitriloside-rich foods. A monograph with references on amygdalin is available at: http://www.worldwithoutcancer.org.uk/therapycom ponents.html#14.
During the late 1970s and early 1980s, I personally was involved in a legal struggle with New York State that involved amygdalin and other alternative cancer therapies. Fortunately for me, I was able to come out of this struggle without much damage to me or my practice. I have been recommending amygdalin as a nutritional supplement for cancer patients since the mid-1970s and believe that it does have value, along with many of the other suggestions and recommendations that we give to our patients.
Chemical Structure of Amygdalin and How It Works
To briefly summarize information about amygdalin and nitrilosides, the following is offered: Amygdalin is one of many nitriloside compounds, which are natural cyanide-containing substances found in many foods, including all of the seeds of the prunasin family (apricots, peaches, apples, pears, and others), millet, buckwheat, cassava melons, and many others. Amygdalin consists of two sugar (glucose) molecules bound to a benzaldehyde, which in turn is bound to a cyanide radical (the benzaldehyde-cyanide radical is called mandelonitrile). Both benzaldehyde and the cyanide radical are potentially damaging to cells, but are quite harmless, while they are bound to the two sugar molecules. As a result of this lack of toxicity while the entire molecule is intact, large quantities of amygdalin (at least 9 grams) can be given as a short intravenous infusion or even as an intravenous push with virtually no side effects or problems.
In the body, the two sugar molecules are split off by the enzyme beta glucosidase (probably by bacteria in the colon) and are replaced by a glucuronic acid molecule to form a compound consisting of glucuronic acid bound to mandelonitrile (the benzaldehyde-cyanide radical). This is actually the true laetrile, according to Krebs, and it differs from the original amygdalin, which has two glucose molecules instead of the glucuronic acid. An enzyme known as beta glucuronidase, which is found in high concentration in cancer cells (but is very scarce in normal cells) splits off the glucuronic acid, leaving benzaldehyde bound to cyanide (mandelonitrile). Once glucuronic acid is split off, the remaining benzaldehyde-cyanide radical spontaneously splits off cyanide, which is toxic to the cancer cell; cancer cells do not have sufficient quantities of any enzyme capable of breaking down or converting the cyanide to a less toxic compound, whereas normal cells do. Hence, there is a selective toxicity to cancer cells.46 Benzaldehyde, like formaldehyde, can also be toxic to cancer cells. So, both benzaldehyde and cyanide are released at the site of the cancer cells and can damage them.
The precise mechanism for amygdalin's selective toxicity to cancer cells but not normal cells involves the protective action of enzymes present in normal cells but lacking in cancer cells. An enzyme present in high concentration in normal cells but very low in cancer cells is the enzyme rhodanese or sulfur transferase. This enzyme transfers a sulfur atom onto the cyanide radical to create the relatively nontoxic thiocyanate. Cancer cells have trouble doing this because they lack this enzyme. So, the cancer cells get the toxic effects of cyanide while the small amount of cyanide released around normal cells is converted to thiocyanate. Blood thiocyanate levels may be used to help monitor the proper dose of amygdalin, helping to make sure that toxic levels of cyanide are not reached but that therapeutic levels are present. Of particular interest is that conventional medicine has used serum thiocyanate to help determine the dosage of their emergency anti-hypertensive drug nitroprusside, a medication that contains a cyanide radical. This drug is still used in emergency rooms for hypertensive crisis. I use the suggested therapeutic range of thiocyanate for nitroprusside to help me monitor appropriate doses of amygdalin.
Studies also indicate that benzaldehyde has anticancer activity, as it combines with cysteine in cancer cells, inactivating various proteins. Cancer cells do not have enzymes capable of converting benzaldehyde. Normal cells, on the other hand, have enzymes capable of oxidizing benzaldehyde to benzoic acid, rendering it harmless to normal cells. Hence, we have a selective toxicity to cancer cells and not normal cells. Incidentally, benzoic acid is converted in the body to hippuric acid, which is discharged in the urine and protects against urinary tract infections.
Clinical Studies and Critiques
Many epidemiological studies, animal studies, and some clinical reports show evidence of amygdalin's efficacy. However, conventional medicine has taken the position that it is useless and/or harmful. It is generally regarded as the height of quackery. Most of the negative views of amygdalin emanate from a study by the late Dr. Charles Moertel of the Mayo Clinic, whose article was published in the New England Journal of Medicine in 1982.47 This is the same Moertel who carried out the studies on vitamin C for cancer that were discussed earlier in this article. He was a long-time opponent of any so-called alternative cancer treatments.
A number of criticisms of this study have been published. A summary of them can be found at: http://www.ispub.com/journal/the_internet_journal_of_alternative_medicine/volume_7_
number_1_22/article/does_laetrile_work_another_look_at_the_mayo_clinic_study_
moertel_et_al_1982.html. (One link, three lines) One issue was that rather than chemically pure amygdalin, a mixture that supposedly mimicked what was being used in a Mexican clinic was used. The patients were treated with IV amygdalin for 3 weeks and then switched to oral amygdalin. During those 3 weeks, 70% of the patients were stable. When they were switched to only oral amygdalin, a large percentage deteriorated. Supporters of amygdalin therapy do not believe that this study proves amygdalin to be useless.
The adult oral dosage for an adult is approximately 500 mg three times daily, but it can be increased or decreased depending upon the patient's clinical response and the results of serum thiocyanate levels. For most adults, the IV dosage that we use is 9 grams, dissolved in a small IV infusion of 100 ml of sterile saline. It is dripped in over 10 to 20 minutes. In our center, we usually administer an IV vitamin C drip and follow it with a drip of amygdalin.
Two cautions should be kept in mind when using amygdalin. First, it is necessary for patients using amygdalin to have a sufficient source of sulfur in the diet so that any excessive cyanide formed near normal cells can be converted to thiocyanate by the addition of sulfur. A relatively inexpensive supplement source is methyl sulfonyl methane (MSM). Secondly, because thiocyanate tends to be suppressive to the thyroid gland, it is essential to have sufficient iodine to overcome any suppression of the thyroid gland by thiocyanate. Iodine as a nutrient with anticancer properties will be discussed below.
Iodine: The Most Misunderstood Nutrient
Iodine supplementation should be considered in all cancer patients. Dr. Max Gerson successfully treated many cancer patients with a variety of unconventional techniques, including more than 10 glasses of raw vegetable juice daily, coffee enemas, a vegan diet, flaxseed oil, cod liver oil, thyroid hormone, and Lugol's solution, which contains relatively high concentrations of iodine.48 Prior to World War II, Lugol's solution was used by numerous physicians worldwide to treat many different conditions. Since then, with the growth of pharmaceutical companies and the widespread use of patentable drugs, inorganic, nonradioactive iodine has not been used for cancer patients or for patients with other disorders who would have previously been treated with iodine.
Guy Abraham, MD, and the Iodine Project
Guy Abraham, MD, former professor of obstetrics, gynecology, and endocrinology at UCLA School of Medicine, has written a series of papers about iodine that has drastically changed my thinking about its role in health and the prevention and treatment of disease. He terms this series of papers "The Iodine Project."
I had been impressed by Abraham's previous work that showed that vitamin B6 and magnesium could be helpful to women with premenstrual syndrome (PMS), and I was eager to learn what he had to say about iodine. Through a series of articles (all of which are available for free download at:
http://www.optimox.com/pics/Iodine/opt_Research_I.shtml), Abraham has proposed that the optimal daily dose of iodine for an adult is approximately 12.5 mg to 50 mg daily, which is close to 100 to 400 times the RDA of 0.150 micrograms (mcg) daily. He believes that the current prevailing medical opinion, that more than 2 mg a day of iodine is toxic, is wrong.
How We Went Wrong: The 'Wolff-Chaikoff Effect'
He traces the source of this mistaken notion about the toxicity of iodine to a scientific experiment on rats that was published in 1948 by Drs. Wolff and Chaikoff.49 From this experiment, the authors erroneously concluded that iodine inhibits the thyroid gland and can cause goiter at doses of about 20 times the recommended daily allowance (RDA) for iodine. This conclusion was indeed surprising, since they had not bothered to check the thyroid hormone levels and actually reported no evidence of an enlarged thyroid (goiter) in any of these rats. This conclusion was reiterated by Wolff again in 1969, and in this paper, he generalized his findings to humans.50 Subsequently, his conclusion was found in medical textbooks, including endocrinology and nutrition textbooks. For example, the Merck Manual online states: "Chronic toxicity may develop when intake is > 1.1 mg/day"51 Even the Linus Pauling Institute on its website says: "The RDA for iodine is sufficient to ensure normal thyroid function. There is presently no evidence that iodine intakes higher than the RDA are beneficial. Most people in the U.S. consume more than sufficient iodine in their diets, making supplementation unnecessary."52 This conclusion, that more than 2 mg of iodine daily in humans can cause hypothyroidism and goiter, is particularly more difficult to understand when as early as 1923, David Marine showed that 9 mg a day of iodine was safe and actually prevented the development of goiter. In a controlled study that used 9 mg of sodium iodide in 2190 students for 2.5 years, Marine found that the incidence of goiter in the group receiving the 9 mg of iodine daily was 0.2% with no evidence of adverse effects, while the control group that did not receive any iodine had an incidence of goiter of 22%.53
How did such an error occur in the original Wolff-Chaikoff experiment? The intent of Wolff and Chaikoff was to determine the effects of inorganic nonradioactive iodine on the thyroid gland in rats. They gave gradually increasing doses of iodine and then used the radioactive iodine uptake test to see what effect these doses had on the thyroid. At that time, the interpretation of the test results was as follows: If the radioactive iodine uptake of the thyroid was suppressed, it was interpreted to mean that the nonradioactive iodine inhibited the thyroid and this phenomenon occurred at what would be about 2 mg of iodine in humans. Since at certain levels of iodine intake the radioactive iodine test showed virtually no iodine uptake, Wolff and Chaikoff decided that the treatment dose of iodine must have inhibited the thyroid gland, and therefore iodine causes hypothyroidism and possibly goiter.
Abraham reinterpreted these findings and concluded that, rather than showing that this amount of iodine inhibited the thyroid, it really showed that this level of iodine made the thyroid gland sufficient in iodine, and therefore it did not need to take up any more iodine. He asserted that the Wolff-Chaikoff interpretation, which became known as the "Wolff-Chaikoff Effect," was absolutely wrong, though it became the focus of attention for the entire health-care industry. According to Abraham, this set back health-care progress for decades.54 Abraham defines and describes the term medical iodophobia as the "unwarranted fear of using and recommending inorganic, non-radioactive iodine/iodide within the range known from collective experience of three generations of clinicians to be the safest and most effective amounts for treating symptoms and signs of iodine/iodide deficiency (12.5 to 50 mg/day)."
Other Reasons for Physicians' Believing that Iodine is Toxic
There are other reasons for the belief among most health-care practitioners that iodine is toxic and dangerous. Allergies to seafood are moderately frequent. A person who is allergic to all seafood or specific seafood such as shrimp will often say that he/she is allergic to iodine because seafood isn't tolerated. The allergy, however, is to a protein or proteins within the seafood (that might or might not contain iodine) and not to iodine itself. The vast majority of patients with seafood allergy can tolerate inorganic, nonradioactive iodine.
Another common allergic reaction occurs when radiographic contrast medium containing iodine is given to a patient for an imaging study and there is an allergic response. The patient is told that he is allergic to iodine when in fact the person is reacting to the whole iodine-containing compound and likely could tolerate nonradioactive, inorganic iodine. However, patients and their clinicians often think that this means the person is allergic to iodine.
Another example involves the antiarrhythmic drug Amiodarone. Although a reasonably effective medication, it is quite toxic and side effects may include death. Every 200 mg tablet of Amiodarone contains 70 mg of iodine, and 9 mg of iodine is released daily over time from the tablet. Medicine has assumed that the toxicity of Amiodarone is due to its iodine content, but a study in 1993 suggests that iodine is the therapeutic component and the rest of the molecule causes the toxic effects.55 Table 6 summarizes the various forms of iodine/iodide used in clinical medicine.
Table 6: Various Forms of Iodine/Iodide Used in Clinical Medicine
Inorganic
• Nonradioactive
-Iodides (e.g., SSKI)
-Tincture of iodine
-Lugol's solution
• Radioactive iodides for diagnostic and therapeutic purposes
Organic
• Naturally Occurring
-Thyroid hormones
-Thyroidal iodolipids
Manmade
-Radiographic contrast media
-Iodine-containing drugs
(e.g., Amiodarone)
Iodine Needed by All Cells and Organ Systems in the Body, Not Just the Thyroid
The commonly accepted medical opinion is that iodine's only role in the body is to help make thyroid hormones. Although this is an extremely important function, Abraham demonstrates that the role of iodine in the body goes far beyond its function of making thyroid hormones. In addition to the thyroid gland, every cell of the body uses iodine. The RDA for iodine of 150 micrograms daily (0.15 mg) is generally sufficient to prevent goiter and to prevent cretinism in infants when the pregnant mother ingests this dosage. However, this dosage is totally insufficient to supply all of the needs of all cells in the body. For example, Finley reported that fibrocystic breast disease could be reversed with 5 mg or more of iodine daily.56 Ghent using 5 mg of iodine daily for a year was able to reverse fibrocystic breast disease in more than 90% of the women in the study.57 Flechas, in a paper at www.optimox.com, says that he can clear fibrocystic breast disease in women within 3 months using 50 mg of iodine daily.58
Other possible functions of iodine include: helping to regulate mood, preventing cancer (especially in breast, ovarian, uterine, prostate, and thyroid gland cancers), helping to regulate blood pressure, helping to regulate blood sugar and prevent and treat diabetes, and helping to prevent abnormal cardiac rhythms. With regard to ancer, in many areas of Japan, Japanese women, who have one of the lowest breast cancer rates in the world, ingest more than 13 mg of iodine daily from seaweed without suffering any adverse consequences; and iodine may be an important factor in this low rate of breast cancer. Abraham further demonstrates that iodine tends to be antibacterial, antiviral, antiparasitic, and antifungal and that it enhances immune function. No microorganism has ever been found to be resistant to iodine. Furthermore, he suggests that suboptimal iodine intake may contribute to various thyroid abnormalities commonly seen today, including hypothyroidism (underactive), hyperthyroidism (overactive) and autoimmune inflammation of the thyroid (Hashimoto's Disease).
Different Forms of Inorganic Iodine/Iodide in the Body and Organ Preferences
Abraham started this Iodine Project around 1998 when he became aware of the many benefits of treating patients with iodine using doses far beyond the 2 mg a day that most physicians consider to be potentially toxic. He noted that, starting in the 1820s, the French physician Jean Lugol used these higher doses to treat a wide variety of conditions. Lugol combined elemental iodine (5%) and potassium iodide (10%) with 85% water. He found that combining the two resulted in elemental iodine being much soluble than when it was used alone. Since iodine kills infectious agents, Lugol successfully treated many infectious conditions with this solution, now known as Lugol's solution, which is still available today by prescription. Prior to World War II, many American and European physicians used Lugol's solution to treat thyroid conditions, using doses higher than 2 mg daily without apparent significant adverse effects.
Abraham notes that research has shown that the thyroid gland prefers to utilize the iodide form of iodine, while other organs, such as the breast and ovaries, prefer the elemental form of iodine.59 Both of these forms are present in Lugol's solution. He points out in his preface to Dr. David Brownstein's book Iodine: Why You Need It; Why You Can't Live Without It:
"Of all the elements known so far to be essential for human health, iodine is the most misunderstood and the most feared. Yet, iodine is the safest of all the essential trace elements, being the only one that can be administered safely for long periods of time to large numbers of patients in daily amounts as high as 100,000 times the RDA. However, this safety record only applies to inorganic nonradioactive forms of iodine … Some organic iodine containing drugs are extremely toxic and prescribed by physicians. The severe side effects of these drugs are blamed on inorganic iodine although studies have clearly demonstrated that it is the whole molecule that is toxic, not the iodine released from it."60
Part 1, Part 3 and Part 4 are also online.
Michael B. Schachter, MD, CNS
Schachter Center for Complementary Medicine
2 Executive Boulevard, Suite 202
Suffern, New York 10901
845-368-4700; fax: 845-368-4727
This article is an expanded version of a lecture presented at the International Society of Integrative Medicine meeting in Tokyo Japan on July 19, 2009. The lecture was partially supported by Maitake Mushrooms Inc. (In the USA, the company is now called Mushroom Wisdom Inc.) The full article was originally published in the International Journal of Integrative Medicine July 2010; 2(1) and is republished in parts in a slightly modified form with permission.

References
25. Jaakkola K et al. Treatment with antioxidant and other nutrients in combination with chemotherapy and irradiation in patients with small-cell lung cancer. Anticancer Res. 1992;12:599.
26. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci. 1976;73(10):3685–3869.
27. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci. 1978;75:4538–4542.
28. Creagan ET, Moertel CG, O’Fallon Jr, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687–690.
29. Moertel CG, Fleming TR, Creagan ET, et al. Highdose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137–141.
30. Pauling, L. Vitamin C therapy and advanced cancer [letter]. N Engl J Med. 1980;302:694.
31. Gonzalez MJ, Rosario-Perez G, Guzman AM, et al. Mitochondria, energy and cancer: The relationship with ascorbic acid. J Orthomol Med. 2010;25(1):29–38.
32. Cameron E, Pauling L. The orthomolecular treatment of cancer. I. The role of ascorbate in host resistance. Chem Biol Interact. 1974;9:273–283.
33. Cameron E, Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974;9:285–315.
34. Cameron E, Campbell A, Jack T. The orthomolecular treatment of cancer. III. Reticulum cell sarcoma: double complete regression induced by high dose ascorbic acid therapy. Chem Biol Interact. 1975;11:387–393.
35. Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609.
36. Hoffer A. Antioxidant nutrients and cancer. J Orthomol Med. 2000;15(4):193–200.
37. Klenner FR. Observations on the dose and administration of ascorbic acid when employed beyond the range of a vitamin in human pathology. J Appl Nutr. 1971;23:61–88.
38. Cameron E, Pauling L. Cancer and Vitamin C. Linus Pauling Institute of Science and Medicine; 1979, 1993.
39. Riordan H, Jackson J, Schultz M. Case study: high-dose intravenous vitamin C in the treatment of a patient with adenocarcinoma of the kidney. J Orthomol Med. 1990;5(1):5–7.
40. Riordan NH, Riordan HD, Meng X, et al. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypothesis. 1995;44(3):207–213.
41. Levine, M. Commentary: Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr. 2000;19(4):423–425.
42. Benade L, Howard T, Burk D. Synergistic killing of Ehrlich ascites carcinoma cells by ascorbate and 3-amino-1,2,4-triazole. Oncology. 1969;23:33–43.
43. Paayatt SJ, Riordan HD, Hewitt, SM, et al. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006;174(7):937–942.
44. Gonzalez MJ, Miranda-Massari JR, Mora EM, et al. Orthomolecular oncology review: ascorbic acid and cancer 25 years later. Integr Cancer Ther. 2005;4(1):32–44.
45. Griffin G Edward. World Without Cancer: The Story of Vitamin B17. Rev ed. Westlake Village, CA: American Media; 1997.
46. Bradford RW, Culbert ML. The Metabolic Management of Cancer: A Physician’s Protocol and Reference Book. Los Altos CA: Robert W. Bradford Foundation; 1979
47. Moertel C, Fleming T, Rubin J, et al. A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. N Engl J Med. 1982;306(4):201–206(s).
48. Gerson MD, Max A. Cancer Therapy: Results of Fifty Cases. Bonita, CA: Gerson Institute; 1990.
49. Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–564.
50. Wolff J. Iodide, goiter and the pharmacologic effects of excess iodide. Am J Med. 1969;47:101–24.
51. Iodine: mineral deficiency and toxicity [Web page]. Merck Manual. August 2008. http://www.merck.com/mmpe/sec01/ch005/ch005e.html.
52. Iodine [Web page]. Linus Pauling Institute Micronutrient Information Center, Oregon State University. Updated March 2010. http://lpi.oregonstate.edu/infocenter/minerals/iodine.
53. Marine D. Prevention and treatment of simple goiter. Alt Med J. 1923;26:437–442.
54. Abraham GE. The safe and effective implementation of orthoiodosupplementation in medical practice. Original Internist. 2004;11:17–36.
55. Phillippou G, Koutras DA, Piperingos G, et al. The effect of iodide on serum thyroid hormone levels in normal persons, hyperthyroid patients, and in hypothyroid patients on thyroxine replacement. Clin Endocr. 1992;37:573–578.
56. Finley JW, Bogardus GM. Breast cancer and thyroid disease. Quart Rev Surg Obstet Gynec. 1960;17:139–147.
57. Ghent W, Eskin B, Low D, et al. Iodine replacement in fibrocystic disease of the breast. Can J Surg. 1993;36:453–460.
58. Flechas JD. Orthoiodosupplementation in a primary care practice. Original Internist. 2005;12(2):89–96. Available at http://www.optimox.com/pics/Iodine/IOD-10/IOD_10.htm.
59. Eskin BA et al. Different tissue responses for iodine and iodide in rat thyroid and mammary glands. Biol Trace Elem Res. 1995;49:9–19.
60. Brownstein D. Iodine: Why You Need It and Can’t Live Without It. West Bloomfield, MI: Medical Alternative Press; 2009.


|
|



![]()
![]()
![]()


