by Garth L. Nicolson, PhD, MD (H)1*,
Robert Settineri, MS2 and Paul C. Breeding, DC3
Abstract
Membrane Lipid Replacement with protected membrane glycerolphospholipids (NTFactor Lipids®) enhanced the bioabsorption of three test nutrients (quercetin, curcumin and coenzyme Q10) in an in vitro Caco-2 gastrointestinal epithelial cell permeability model. In vivo oral use of NTFactor Lipids® in an open label study showed reduced multiple, self-reported symptoms in chemically exposed Gulf War veterans with multi-symptom chronic illnesses. In the patients that fully complied and completed a six-month study there were gradual and significant reductions of symptom severities in categories related to fatigue, pain, musculoskeletal, nasopharyngeal, breathing, vision, sleep, balance, urinary, gastrointestinal and chemical sensitivities. Membrane Lipid Replacement with protected membrane glycerolphospholipids is a simple, safe and potentially effective method of slowly reducing the severities of multiple symptoms in chemically exposed veterans. In addition to potentially promoting the increased absorption and transport of nutrients through intestinal epithelial cells, Membrane Lipid Replacement has its own health benefits in reducing symptom severities in chronically ill patients.
Introduction
Membrane Lipid Replacement (MLR) using dietary protected polyunsaturated glycerolphospholipids, such as NTFactor Lipids®, results in the systemic replacement of damaged cellular membrane glycerolphospholipids with undamaged, unoxidized lipids to ensure
the proper function of cellular membranes, such as mitochondrial membranes.1-3 By blending the glycerolphospholipids with antioxidants and membrane-protecting fructooligosaccharides, MLR supplements have proven to be effective in reducing multiple, chronic illness symptom severities and age-associated loss of function, while providing mitochondrial and other membrane support.1-5 The MLR supplement NTFactor Lipids® has been used in several clinical studies that demonstrate its safety and efficacy.3-7 Here we show that NTFactor Lipids® can improve nutrient transport in an intestinal epithelial bioabsorption model.8 In addition, in chemically exposed veterans, this supplement can reduce the severity of several chronic signs and symptoms.9
MLR and Intestinal Bioabsorption Models
Prior to clinical studies on the effects of various nutrients on health indicators, cell-based assays are often used to provide evidence for human intestinal absorption and bioavailability of oral supplements.10 In vitro cultured cell models have been used for epithelial absorption and bioavailability, and these have been found to compare well with ex vivo organ absorption.10,11 In such in vitro models various lipids have been added to oral supplements to enhance epithelial cell absorption and bioavailability.12 Although other factors can affect gastrointestinal transit, digestion, dissolution and epithelial absorption, this last event is critical for the overall bioavailability of oral nutrients.12
One of the most widely used cell models for bioabsorption and bioavailability is based on the human colon epithelial cell line Caco-2.13 The differentiated Caco-2 epithelial cells are used in polarized monolayer cell cultures to mimic an epithelial cell barrier.8-11 Caco-2 cell monolayers express a wide range of properties that mimic an intestinal epithelial cell layer and have been used as uptake/bioavailability models.9,10 In these models Caco-2 intestinal monolayers are grown on a supportive membrane surface that separates two compartments in a trans-well where test substances can be sampled.11,13 Caco-2 uptake studies have been used as the reference standard in the pharmaceutical and nutraceutical industries for the in vitro prediction of in vivo human intestinal absorption and bioavailability of orally administered nutrients.11,13,14
We used the Caco-2 intestinal monolayer test system to see if NTFactor Lipids® can improve nutrient transport. First, the reliability of the Caco-2 test system was confirmed in a control study.8 The results indicated that the trans-well permeability system worked as expected during the assay. A low-permeability molecule, talinolol, control showed a Papp value of <0.5, and a high permeability molecule, warfarin, control was found to have a Papp value of >100, as expected.8
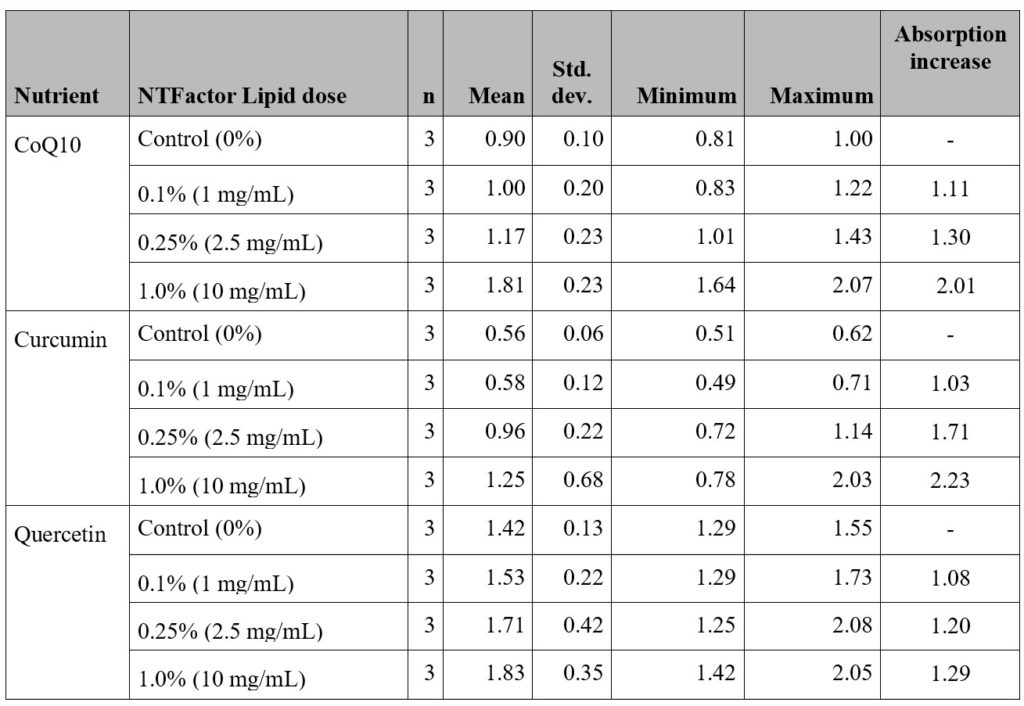
and with different concentrations of NTFactor Lipids® using
the Caco-2 test system (n=3). (Data from Settineri et al.8)
For studying the effects of MLR on bioabsorption and bioavailability of test nutrients, each nutrient was tested with three concentrations of NTFactor Lipids® (provided by Nutritional Therapeutics, Inc.).8 For each nutrient, the mean response versus dose level and the standard deviation for the mean are shown in Table 1. For example, in Caco-2 bioabsorption studies of CoQ10 (with 0 to 1% NTFactor Lipids®) transport was increased 2.01-times over controls with 1% NTFactor Lipids® (p= 0.0011).8 Although there were positive dose-dependent responses of curcumin and quercetin absorption with NTFactor Lipids®, these did not reach the same level of significance as seen with CoQ10.8 However, when the overall combined dose increase was compared with all three nutrients, ANOVA analysis indicated a significant collective enhancement of absorption by NTFactor Lipids® (p< 0.001).8
The above study demonstrated that the bioavailability of nutrients, especially those with more lipophilic structures, when combined with NTFactor Lipids®, should result in increased absorption and transport through intestinal epithelial cells compared to the nutrients without added glycerolphospholipids. This suggests strongly that NTFactor Lipids® can promote oral nutrient bioavailability.8
Effects of NTFactor Lipids® on Symptom Severities in Chemically Exposed Veterans
Many veterans of the first Gulf War in 1991 returned and slowly displayed multiple signs and symptoms related to their deployment.15,16 The most common signs and symptoms found included chronic fatigue, arthralgia, myalgia, headaches, gastrointestinal problems, sleeping difficulties, dermatological symptoms, breathing problems, loss of concentration and cognition, depression, muscle spasms, nervousness, blurred vision, anxiety, chest and heart pain, dizziness, nausea, stomach pain, loss of balance, hives, frequent coughing, chemical sensitivities, eye pain, vision problems and photophobia, bleeding gums, and other symptoms.15-18 This multi-symptom chronic illness has been called Gulf War Illness or Gulf War Illnesses (GWI).19,20 Many of these veterans appear to have their diagnoses linked to chemical exposures, such as oil spills and fires, smoke from military operations, chemicals on clothing, pesticides, chemoprophylactic agents (pyridostigmine bromide), chemical weapons and other possible exposures.18-22 However, the apparent spread of a similar illness to immediate family members in the absence of environmental exposures suggests that a minority of veterans were exposed to an infection(s).19,23,24 In addition, fine sand exposure was rather ubiquitous during deployment, and continued pulmonary exposure to fine sand particles can result in hyperergic lung conditions and pneumonitis.25 The exposures to different, multiple agents and toxicants have made the successful treatment of GWI quite difficult.19,26
Treatment of GWI has been complicated by multiple toxic exposures (chemical, biological, and in a few cases radiological as well as stress) and the lack of available methods to successfully treat or remove offending substances.19,26 MLR has been used to slowly remove hydrophobic toxic molecules, and possibly some of the toxic chemicals implicated in some cases of GWI.19,21,26, This removal process is thought to occur by a concentration-dependent process, driven by a bulk flow or mass action mechanism. Hydrophobic molecules are slowly sequestered into small glycerolphospholipid droplets, vesicles and other structures and eventually removed from cells and tissues by mass action, transported by the blood circulation, and deposited into the gastrointestinal system for elimination.27,28
Most uses of MLR do not require the higher doses of NTFactor® or NTFactor Lipids® (usually 2-4 g per day) to affect the severity of multiple signs and symptoms. For example, several studies on various clinical conditions and aging have used the 2-4 g per day dose range.3,4 However, to drive the removal of hydrophobic, toxic chemicals and support the health of Gulf War veterans higher dose levels were required (6 g per day) in case studies.29,30 Also, most non-GWI clinical studies indicated that reductions in symptom severities found with MLR occurred much sooner than found in most cases of GWI. Although the reasons for this are not clear at this time, they may be related to deeply embedded, toxic chemicals in veterans that appear to be difficult to remove and are only removed slowly from their hydrophobic sites.9
Case reports previously suggested the usefulness of MLR in Gulf War veterans to lower symptom severities and improve health.29,30 In these case reports the daily dose of NTFactor Lipids® was increased to 6 g per day in order to improve the rate of removal of toxic chemicals. Thus an open-label, Institutional Review Board-approved, preliminary clinical study was initiated to study the clinical effects of a glycerolphospholipid chewable wafer supplement containing NTFactor Lipids® (provided by Nutritional Therapeutics, Inc.) on the severities of signs and symptoms of GWI patients. Five supplement wafers providing a total of 6 g of NTFactor Lipids® were taken each day for six months, and various signs and symptoms were self-reported at various times (0, 0.25, 1, 3 and 6 months) using a validated patient symptom survey form.9 The symptom severities in the survey form were scored numerically based on a linear scale from 0 to 10 or lowest (0) to highest (10) severity of symptoms. The symptom severity survey form contained approximately 120 signs and symptoms that were later merged into 22 symptom categories to more easily assess the effects of NTFactor Lipids®.9 The patients were male US Marines and US Army veterans who were chemically exposed during their deployment, had been ill since their deployment without relief, and had signed informed consent documents. The average age in years of participants that fully completed the study was 53.1±3.2. Each patient was diagnosed with GWI based on their deployments and diagnosis of Gulf War Illness using the chronic multi-symptom illness standards developed by the US Government and the State of Kansas.15,31 Inclusion criteria in the study included at least three of six symptom categories: fatigue, pain, neurological, skin, gastrointestinal, respiratory symptoms.31 Exclusion criteria included diagnosis of a serious medical condition not usually associated with deployment to the Persian Gulf region or a psychiatric condition that could account for some symptoms, or an illness that could interfere with accurate scoring of sign/symptom severity.9

Study participants were exposed to a variety of environmental conditions and toxicants in the Persian Gulf region.9 For example, all of the participants reported exposures to oil well fires and burn pit smoke, fuels, and crude or refined oils (Figure 1).9 In addition, all of the participants took oral chemoprophylactic agents (pyridostigmine bromide) during the conflict. Most participants reported that they were also exposed to pesticides, but only a few to insects. Some trial participants also reported exposure to raw sewage and dead bodies.9 A few participants may have been exposed to chemical warfare agents, and there was a very low incidence of reported exposure to herbicides (Figure 1).9
At the beginning of the study, veterans reported a variety of signs and symptoms severities that were unique for each patient. In Figure 2 the self-reported symptoms and severities of the study participants were grouped into symptom categories, and the reported category severities and their change over time were different and collected for each patient.9 The mean changes in the study group’s self-reported symptom categories with time during the study are shown in Figure 2 along with the standard deviations of symptom severities. Although there were differences in the symptom category severities and responses to the test supplement in individual subjects, the group mean data indicated that there were gradual and significant responses to the test supplement. Specifically, there were significant reductions in symptom category severities related to fatigue, pain, musculoskeletal, nasopharyngeal, breathing, vision, sleep, balance, gastrointestinal, chemical sensitivities and other symptom categories (Figure 2).9
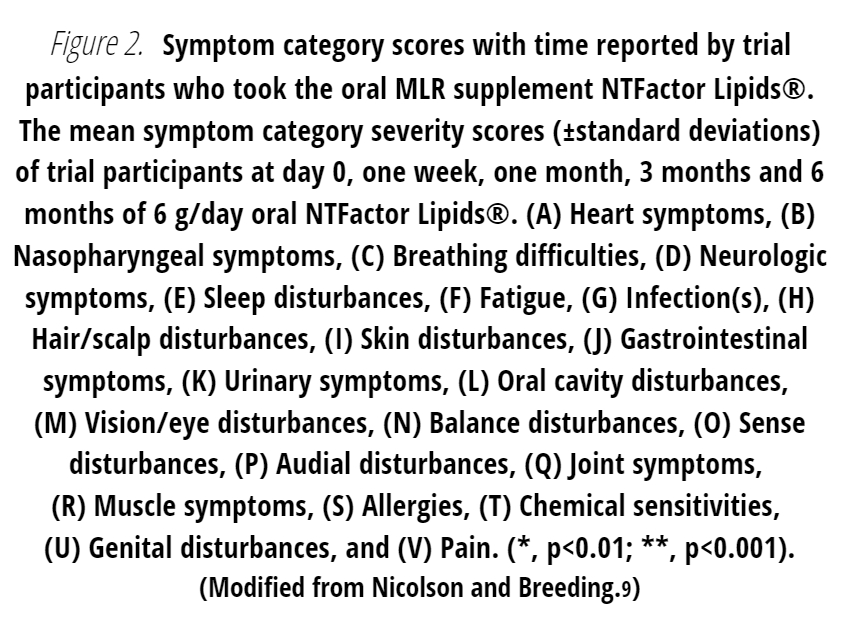
As expected, the reductions in certain symptom severities were gradual and significant (p<0.01 to p<0.001) with time as shown in various symptom categories, such as fatigue (Fig. 2F), sleep disfunction (Figure 2E), gastrointestinal symptoms (Figure 2J), pain (Figure 2V) and other symptoms. In the present study we also considered self-reported changes in chemical sensitivities (Figure 2T). In general, there were significant reductions in certain symptom categories that have been related to GWI.9
Discussion
We have briefly presented some recent data on the use of MLR with the dietary supplement NTFactor Lipids®. This supplement has the structure and properties to improve the transport and bioabsorption of many nutrients as well as enhance cellular energy by repairing free radical damage to mitochondrial and other cellular membranes.1-8 MLR with NTFactor Lipids® has been used to reduce symptom severity in patients with fibromyalgia, chronic fatigue, chronic pain and other chronic conditions that have pain and/or fatigue as major symptoms.1-7 Some symptoms, such as fatigue and pain, also occur during normal aging, and they are important as secondary symptoms in many if not most chronic diseases.32-34
MLR with oral glycerolphospholipids have been used successfully in several clinical studies to reduce symptom severity and improve quality of life assessments.1-7 The MLR supplement NTFactor Lipids® with fructooligosaccharides (to protect the phospholipids from disruption, degradation and oxidation in the gut) and antioxidants have significantly reduced symptom severity in various chronic illness patients (see reviews1-7). The membrane glycerolphospholipids are quickly and almost completely absorbed and transported into tissues and cells without excessive oxidative damage.2,3 There the undamaged, replacement membrane phospholipids can exchange with damaged membrane phospholipids, resulting in replacement of the damaged molecules and in the process also remove other hydrophobic molecules. In addition, MLR glycerolphospholipids provide important precursors for specific membrane molecules, such as mitochondrial cardiolipin.2,3
Oral MLR supplements such as NTFactor Lipids® have been designed to reduce fatigue and protect cellular and especially mitochondrial membranes from damage.1-7 By combining NTFactor Lipids® with vitamins and minerals (such as PropaxTM with NTFactor Lipids®), cancer patients have shown reductions in the adverse effects of cancer therapy, such as chemotherapy-induced fatigue, nausea, vomiting and other adverse side effects.33 MLR with NTFactor Lipids® have also been used in other illnesses to reduce symptoms, especially fatigue.1-7 One combination supplement, ATP Fuel (Researched Nutritionals, Inc.) with NTFactor®, CoQ10, NADH and other nutrients has been used to significantly reduce fatigue in patients with intractable chronic fatiguing illnesses.34 Indeed, the use of NTFactor® and NTFactor Lipids® in conjunction with several other natural supplements to restore mitochondrial function has many potential uses.35 Finally, the most universal use of MLR supplements like NTFactor Lipids® may be as a completely safe anti-aging supplement to increase the performance of cellular membranes, especially mitochondrial membranes, that functionally decline with advanced age.2-7,30
Patient Consent
The data collection and patient evaluations were approved by an independent institutional review board. Patients consented to use their clinical information via informed consent.
Acknowledgements and Disclosures
The authors would like to thank Nutritional Therapeutics, Inc. and the Institute for Molecular Medicine for financial support and for providing study materials. Garth L. Nicolson is a part-time consultant to Nutritional Therapeutics, Inc and Naturally Plus Taiwan, Robert Settineri is a part-time consultant to Nutritional Therapeutics, Inc., and Paul C. Breeding has no potential conflicts to disclose.
References
- Nicolson GL. Membrane Lipid Replacement: clinical studies using a natural medicine approach to restoring membrane function and improving health. Intern J Clin Med. 2016; 7: 133-143.
- Nicolson GL, Ash ME. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim Biophys Acta Biomemb. 2017; 1859: 1704-1724.
- Nicolson GL, Ferreira de Mattos G, Ash M, et al. Fundamentals of Membrane Lipid Replacement, a natural medicine approach to reducing fatigue, pain, and other symptoms while restoring function in chronic illnesses and aging. Membranes. 2021; 11(12): article 944.
- Nicolson GL, et al. Clinical uses of Membrane Lipid Replacement supplements in restoring membrane function and reducing fatigue in chronic diseases and cancer. Discoveries. 2016; 4(1): e54.
- Agadjanyan M, et al. Nutritional supplement (NTFactor) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. J Chronic Fatigue Syndr. 2003; 11(3): 23-36.
- Nicolson GL, Ellithrope RR. Lipid replacement and antioxidant nutritional therapy for restoring mitochondrial function and reducing fatigue in chronic fatigue syndrome and other fatiguing illnesses. J Chronic Fatigue Syndr. 2006; 13(1): 57-68.
- Nicolson GL, Settineri R, Ellithorpe R. Neurodegenerative and fatiguing Illnesses, infections and mitochondrial dysfunction: use of natural supplements to restore mitochondrial function. Funct Foods Health & Dis. 2014; 4(1): 23-65.
- Settineri R, et al.. The effects of Membrane Lipid Replacement with NTFactor® Lipids on increasing the bioavailability of three test nutrients. Bioact Comp Health Dis. 2022; 5(5): 106-116.
- Nicolson GL, Breeding PC. Membrane Lipid Replacement with glycerolphospholipids slowly reduces self-reported symptom severities in chemically exposed Gulf War veterans. Intern J Transl Med. 2022; 2(2): 164-173.
- Schoultz I, Keita AV. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. 2020; 9(8): 1909.
- Fedi A, et al. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: a systematic review. J Contr Release. 2021; 335: 247-268.
- Rezhdo O, Speciner L, Carrier RL. Lipid-associated oral delivery: mechanisms and analysis of oral absorption enhancement. J Contr Release. 2016; 240: 544-560.
- Fuch J, Orfeo T, Tiso J, Sharkey FE. Establishment of human colon carcinoma lines in nude mice. Pathobiol. 1979; 47: 136-144.
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of human colon carcinoma cell line (Caco- 2) as a model system for intestinal epithelial permeability. Gastroenterol. 1989; 96: 736-749.
- Fukuda K, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998; 280(11): 981-988.
- Kang, HK. et al. Illnesses among United States veterans of the Gulf War: a population-based survey of 30,000 veterans. J Occup Environ Med. 2000; 42(5): 491-501.
- Doebbeling BN, et al. Is there a Persian Gulf War syndrome? Evidence from a large population-based survey of veterans and nondeployed controls. Am J Med. 2000; 108(9): 695-704.
- Proctor SP, et al. Health status of Persian Gulf War veterans: self-reported symptoms, environmental exposures and the effect of stress. Intern J Epidemiol. 1998; 27(6): 1000-1010.
- Nicolson GL, et al. Gulf War Illnesses: chemical, radiological and biological exposures resulting in chronic fatiguing illnesses can be identified and treated. J Chronic Fatigue Syndr. 2003; 11(1): 135-154.
- Yee MK, et al. Multiple mild traumatic brain injuries are associated with increased rates of health symptoms and Gulf War illness in a cohort of 1990-1991 Gulf War veterans. Brain Sci. 2017; 7(7): 79.
- White RF, et al. Recent research on Gulf War Illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016; 74: 449-475.
- Golomb BA. Acetylcholinesterase inhibitors and Gulf War Illnesses. Proc Natl Acad Sci USA. 2008; 105(11): 4295-4300.
- Nicolson GL, et al. High prevalence of mycoplasmal infections in symptomatic (Chronic Fatigue Syndrome) family members of mycoplasma-positive Gulf War Illness patients. J Chronic Fatigue Syndr. 2003; 11(2): 21-36.
- Nicolson GL, et al.. High frequency of systemic mycoplasmal infections in Gulf War veterans and civilians with Amytrophic Lateral Sclerosis (ALS). J Clin Neurosci. 2002; 9: 525-529.
- Korényi-Both AL, et al. Al-Eskan disease: Desert Storm pneumonitis. Mil Med. 1992; 157(9): 352-462.
- Hodgson MJ, Kipen HM. Gulf War Illnesses: causation and treatment. J Occup Environm Med. 1999; 41(6): 443-452.
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993; 121: 1257–1269.
- Nicolson GL, Ferreira de Mattos G. A brief introduction to some aspects of the Fluid—Mosaic Model of membrane structure and its importance to Membrane Lipid Replacement. Membranes. 2021; 11(12): article 947.
- Nicolson GL, Breeding PC. Membrane Lipid Replacement for reduction of pain, fatigue, gastrointestinal and other symptoms in patients with peripheral pain: case reports. Case Rep Rev. 2020; 1(2): 1-3.
- Nicolson GL, et al. Aging and chronic illnesses: Membrane Lipid Replacement for restoring mitochondrial function and reducing fatigue, pain, and other symptoms in aged individuals. Bioactive Comp Health Dis. 2020; 3(10): 194-203.
- Steele L. Prevalence and patterns of gulf war illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemol. 2000; 152: 992-1002.
- Reid S, et al. Chronic fatigue syndrome. BMJ Clinical Evid. 2011; 2011: 1101.
- Nicolson GL. Lipid replacement therapy: a nutraceutical approach for reducing cancer-associated fatigue and the adverse effects of cancer therapy while restoring mitochondrial function. Cancer Metastasis Rev. 2010; 29(3): 543-552.
- Nicolson GL, Settineri R, Ellithorpe E. Lipid Replacement Therapy with a glycophospholipid formulation with NADH and CoQ10 significantly reduces fatigue in intractable chronic fatiguing illnesses and chronic Lyme disease. Intern J Clin Med. 2012; 3(3): 164-170.
- Gonzalez MJ, et al. Mitochondrial correction: a new therapeutic paradigm for cancer and degenerative diseases. J Orthomol Med. 2018; 33(4): 1-20.