By Pushpa Larsen, ND
Back in what is now the early days of the pandemic, reports started to surface of a symptom that at the time was being referred to as “COVID toes.” That, along with other reports of microcirculatory impairment and thrombotic microangiopathy,1 led me to wonder about the relationship between blood viscosity and COVID-19 infection. Does an elevated blood viscosity predispose an individual infected with the SARS-CoV-2 virus to more severe infection and poorer outcome? Does the SARS-CoV-2 virus, itself, increase blood viscosity? Can optimizing blood viscosity be helpful in improving outcomes and decreasing the duration of illness? Or are they simply coincidental? More recently, a colleague speculated that the phenomenon known as “long COVID” may also have a relationship to blood viscosity.
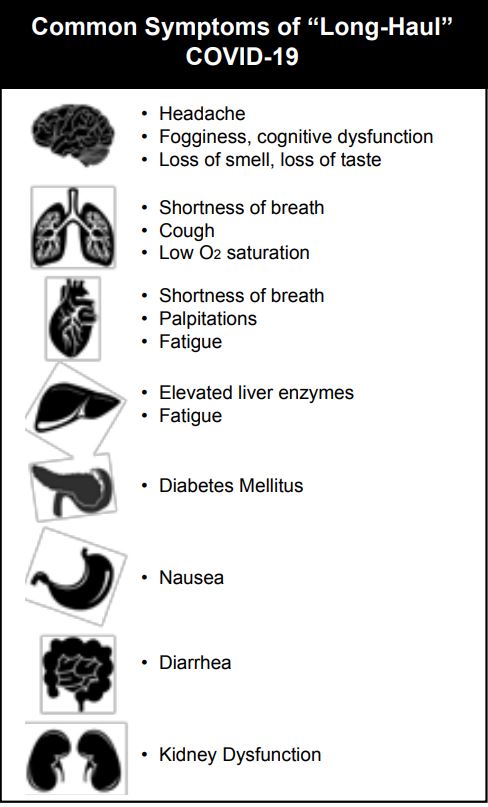
According to a large retrospective study of 273,618 COVID-19 survivors by Oxford University, long-COVID occurs in more than one-third of patients.2 Symptoms of long-COVID are diverse and can include any organ system in the body. Long COVID may manifest as neurocognitive disorders, mental health disorders, metabolic disorders, and disorders of the cardiovascular, gastrointestinal, and musculoskeletal systems. Malaise, fatigue, muscle and joint pain, and anemia have all been reported.3-6 In October 2021, the World Health Organization (WHO) issued a report attempting to define long-haul COVID, which they term “Post COVID-19 Condition,” as a clinical entity that “occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.”7 Cognitive dysfunction, often described as “brain fog” and fatigue are among the most commonly reported symptoms that negatively impact daily functioning.
A Short Review of Blood Viscosity
When I first started studying and writing about blood viscosity in 2012, I found that there was a wide variety and number of conditions associated with elevated blood viscosity, including most conditions associated with aging, such as hearing loss, vision loss, arthritis, and osteoporosis.8-15 This is not actually surprising when you consider how blood viscosity affects perfusion.
Blood viscosity, you may recall, is the thickness and stickiness of blood. A whole blood viscosity test is a functional assay that directly quantifies the resistance of blood to flow and is a biophysical property of the blood that effectively aggregates all of the cellular content and plasma proteins to assess how the blood resists or responds to the life-sustaining forces of perfusion that are powered by the human heart. Taken together, the blood is the largest organ in the human body by volume. Blood viscosity directly determines how hard the heart has to work as well as perfusion to each cell, tissue, and organ; Five primary factors are most influential in determining blood viscosity: Hematocrit, erythrocyte deformability (or rigidity), plasma viscosity, erythrocyte aggregation, and temperature.16
Hematocrit, of course, directly influences whole blood viscosity because a greater proportion of erythrocytes results in thicker, more viscous blood. Red blood cell deformability refers to the ability of erythocytes to flex and change shape. The average red blood cell has a diameter of about 7.5-8.7µm, or about double that of the average capillary, which has a diameter of 3-4µm. The ability of RBCs to bend and fold is crucial to making their way through the capillary beds. This is where perfusion to the organs and tissues takes place. Less flexible RBCs result in more viscous blood and poorer perfusion.
Plasma viscosity refers to the thickness or viscosity of the blood after the cellular material has been removed. Plasma viscosity is highly affected by hydration and plasma proteins, especially high-molecular weight proteins such as immunoglobulins and fibrinogen, and is a third factor determining whole blood viscosity. Diagnostic tests for plasma viscosity are available in most hospitals but are not commonly ordered outside the hospital setting. The fourth factor is erythrocyte aggregation or the tendency of red blood cells to be attracted to each other and stick together. Finally, body temperature impacts blood viscosity, although to a much lesser extent than the other four factors. As a rule of thumb, a 1°C increase in temperature results in a 2% decrease in whole blood viscosity.
Although blood viscosity plays a role in plaque formation in the arteries, the greater impact may be how it effects microcirculation. Hyperviscosity of the blood directly impacts the brain and every other organ system in the body at the microperfusion level. Non-deformable erythrocytes have a diminished ability to pass through capillaries, reducing oxygen delivery and causing abrasion of capillary walls. The body responds by thickening the capillary walls, further decreasing diffusion of oxygen and nutrients into the tissues and of CO2 and waste products back into the blood for removal. This effect is most pronounced in those tissues where healthy capillaries are essential for unimpaired function, such as the brain, kidneys, eyes, and the fingers and toes.
It is worth noting that most of the medications that are referred to as “blood thinners”are more properly called anticoagulants. They do not decrease blood viscosity, as they do not decrease hematocrit, make RBCs more flexible, improve hydration, etc. Anticoagulants simply make it take longer – sometimes much longer – for the blood to clot. So patients on warfarin or other anticoagulants can still have viscous blood that is unable to fully perfuse the tissues.
How Does All of This Relate to COVID-19?
It was clear from very early on during this global pandemic that risk of severe COVID infection had some association with age. Countless reports from clinical researchers and the medical news have underscored that children and young adults may be asymptomatic or far less prone to severe illness, whereas the elderly are at particularly high-risk for more severe COVID infections, symptoms, and acute respiratory distress. Whole blood viscosity also has a direct positive correlation with patient age.
More than 20 years ago, researchers at the National Institute of Aging performed a clinical study randomly selecting 147 subjects from the Baltimore Longitudinal Study of Aging and evaluated age-associated changes in cerebral blood flow. While other rheologic measures like plasma viscosity, red cell rigidity, mean cell volume, red cell aggregation, and fibrinogen showed significant linear increases with age, (Figure 1), whole blood viscosity demonstrated an altogether different trend. The relationship between blood viscosity and age remained relatively unchanged, until after age 60, when blood viscosity was shown to increase exponentially with age. (Figure 2) These changes coincided with age-associated decreases in blood flow and flow velocity as confirmed with Doppler ultrasonography.17
Traditional cardiovascular risk factors, such as hypertension, obesity, diabetes and metabolic syndrome as well as cardiovascular events have all been strongly linked with hyperviscosity of blood; and these are also pre-existing conditions associated with the severity of COVID infections and complications in COVID-19. In a prospective study, 331 hypertensive men were followed for up to 12 years after measuring diastolic blood viscosity (i.e., low-shear rate viscosity). Patients with hyperviscosity had more than threefold greater CVD events than those with reduced viscosity (hazard ratio=3.42, 95% confidence interval=1.4–8.4, p=0.006).18 In a study of 128 obese people (BMI > 28) and 90 non-obese healthy controls, diastolic blood viscosity was 15% higher in obese vs. non-obese patients.19 Historically numerous studies have also shown type-II diabetics have higher systolic and diastolic viscosity than healthy non-diabetics20; patients with metabolic syndrome have higher viscosity than those without21; and baseline viscosity scores are predictive of incident diabetes in initially non-diabetic adults.22 A number of outcome studies have demonstrated the risk of major CVD events increased with blood viscosity,23 and stroke patients and those with stroke risk factors were shown to have elevated blood viscosity relative to healthy controls.24
Neurocognitive problems have been shown to be directly correlated to whole blood viscosity as was reported in the Edinburgh Artery Study.25 The Edinburgh Artery Study was the largest clinical study of blood viscosity ever done; and among a number of other interesting insights, it found that whole blood viscosity was predictive of cognitive decline over a four-year period in 452 elderly subjects (p<0.05). The researchers used a number of established tests, such as the Wechsler Logical Memory Test for verbal memory, the Wechsler Digit Symbol Test for processing speed, Raven’s Progressive Matrices for non-verbal reasoning, and a phonemic Verbal Fluency Test, and found whole blood viscosity was associated with decreases in general cognition (p<0.05), non-verbal reasoning (p<0.05) and information processing speed (p<0.01).
In a December 2020 short review, Østergarrd tied symptoms experienced by those with both acute and “long covid” to impaired microcirculation and tissue hypoxia. Mulitple mechanisms were described, several of which would affect whole blood viscosity.26
No clinical study has yet been published directly connecting whole blood viscosity to COVID complications or severity of the disease. But plasma viscosity, which is a component of whole blood viscosity, was shown, in a series of 15 critically ill patients admitted to the intensive care unit with COVID-19 pneumonia and treated with anticoagulation, to exceed the 95th percentile in all 15 cases.27 This study, conducted at Emory University, demonstrated that plasma viscosity was highly correlated with Sequential Organ Failure Assessment scores (r=0.841, p<0.0001). Measuring viscosity of plasma (having cellular content removed) or serum (clotting factors also removed) is far more common than whole blood viscosity, but it is the whole blood viscosity that more accurately reflects states of ischemia/perfusion/hemorrhage, as it includes the cellular material and interactions, and can serve as a biomarker with wide dynamic range for risk stratification.
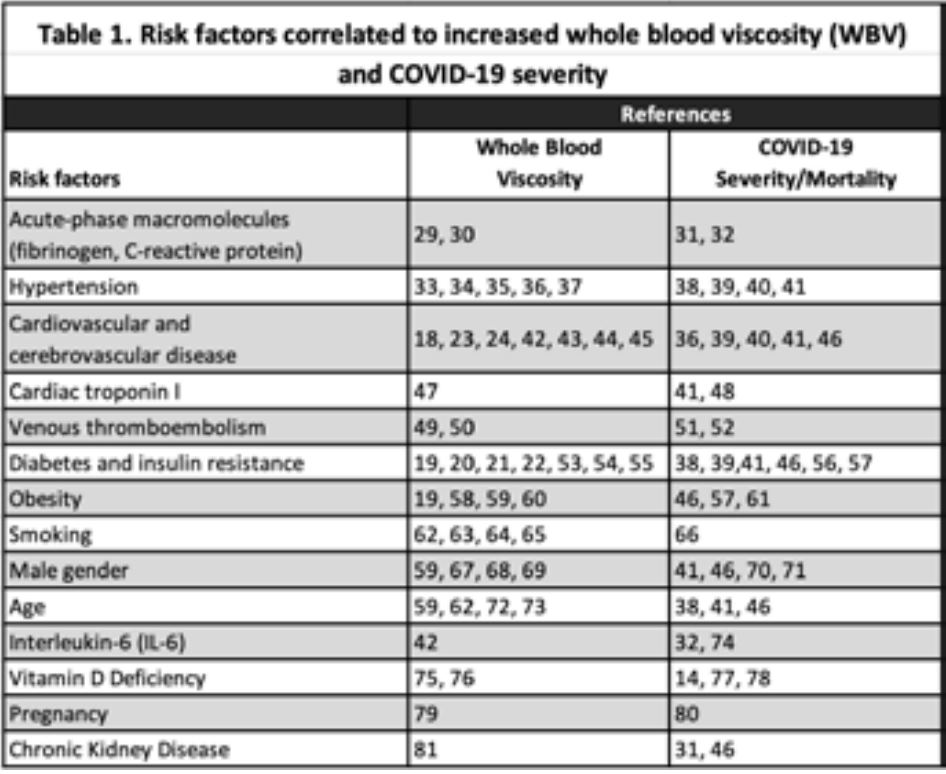
Table 1 enumerates associations between whole blood viscosity and a range of risk factors and the severity of COVID infection with the same risk factors. No causal links can be assumed from these observations, but they are interestingly consistent. I am also not suggesting that whole blood viscosity can be used to stratify COVID patients to predict mortality or the severity of the disease. There isn’t adequate data to support that at this time. It does seem to be increasingly clear, however, that blood viscosity is an important biomarker in assessing the health of our patients. Optimizing blood viscosity may be a strategy to improve symptoms in COVID long-haulers. Certainly, it can be hypothesized that improving perfusion to all body tissues should help with fatigue. Improving perfusion to the brain may also improve the memory loss and “brain fog“ so frequently experienced by long-haulers. In a 2021 article in Clinical Hemorheology and Microcirculation, Paolo Luis Farber concludes, “. . .many studies are suggesting that comorbidities linked to severe covid-19 are also related to erythrocyte factors that increase blood viscosity in microcirculation, such as erythrocyte aggregation and erythrocyte deformability.”28