…article continued:
In support of the concept that saliva and capillary blood levels of hormones with topical physiological dosing more accurately represent tissue uptake and response than serum blood or urine levels, studies have evaluated target tissue levels of hormones and tissue response to the topical delivery of the sex hormones E2 and Pg in human breast and uterine tissues.8,17 Chang and coworkers looked at the levels of E2 and Pg in breast biopsies and tissue response (increase or decrease in mammary cell proliferation) following topical E2 (1 mg) and Pg (25 mg) application directly to the breasts of women.8 Mammary tissue levels of hormones, determined by breast biopsy following therapy with topical E2, Pg, or placebo showed a 100-fold increase in breast tissue E2 and Pg and marked Pg inhibition of E2-stimulated mammary cell proliferation. Not surprisingly serum levels of E2 and Pg in these women showed no significant change from patients treated with topical E2 and Pg vs a placebo topical cream. Similar results have been reported for physiological dosing of Pg (30 mg) that protected the uterus from overstimulation by conjugated estrogens and transdermal E2.17,18 These data are in line with studies2,3 and Tables 1-2, showing that physiological doses of topical E2 and Pg raise salivary and DBS levels of E2 and Pg to high physiological levels but have little impact on serum or urine levels of these hormones.
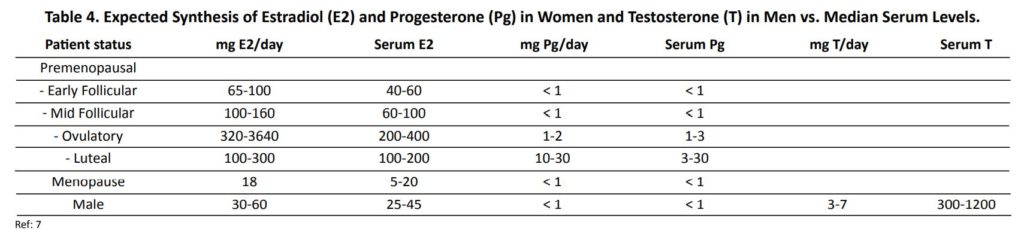
Topical hormone delivery is often described as a “first-pass” type of delivery meaning that it circumvents the liver when it first enters the bloodstream and in doing so does not induce steroid binding proteins (SHBG, CBG, and TBG) or increase clotting factors. However, as we and others have found, in the context of topical hormones “first-pass effect” is more likely a misnomer because even with continued long term use, topically delivered hormones never increase to any significant extent in serum or urine, or affect liver synthesis of hormone binding globulins, in contrast to what is seen when the liver is exposed to high levels of circulating estrogens with other forms of estrogen therapy (e.g., oral, intramuscular/subcutaneous, pellet, troche, sublingual). Based on the evidence that progesterone never accumulates in serum or urine with prolonged therapy2 (Table 2), a more appropriate description for topical sex hormone therapy, at least for progesterone, would be a “no-pass effect.”
What is the evidence that topically delivered hormones might transmigrate through the skin into the lymphatics instead of the blood vascular system? From pharmacokinetic studies2 we know that when a steroid hormone is delivered as a cream or gel to the surface of the skin, it takes about two-to-six hours before it peaks in saliva or capillary blood from the fingertip, and then begins to slowly decline to baseline within 12-36 hours. If hormones were passing from the skin into the blood vascular system directly, levels in serum and capillary blood would be near equivalent as seen for endogenous production of these hormones, and salivary levels would be about 2% of blood levels,. They are not. Saliva and fingerstick blood represent hormones present in the interstitial fluid of capillary beds. Venipuncture blood serum and urine are more representative of hormones outside the capillary beds and interstitial tissue.
These clues and emerging research into topical sex hormone delivery into different body fluids strongly suggest that the sex steroids mostly are not being delivered to tissues by the blood vascular system, but instead point to another system of delivery, the most likely of which is the lymphatics, as elaborated by others.3,15 If correct, this might suggest that topical hormone therapy will be more beneficial for tissues with high lymphatic infiltration, such as the reproductive tissues (breasts, uterus), brain, and immune system, all targets for female hormone therapies. It might also mean that tissues with less lymphatic infiltration, such as the bones and skeletal muscle,19 would receive less hormone and be affected less. If so, topically delivered sex hormones such as E2 and Pg would be less effective in promoting bone growth in postmenopausal women suffering from osteoporosis, and T would be less effective in increasing muscle mass in men and women suffering from sarcopenia caused by androgen deficiency.
That the breasts and uterus are heavily infiltrated by lymphatics and the brain by the glymphatic system is consistent with the effectiveness of physiological sex hormone replacement therapy in these tissues and for physiological (10-30 mg) Pg dosing being able to counter the growth-promoting actions of estrogens in the breasts8 and uterus17,18 without raising serum levels of Pg (Table 2). Glymphatic delivery of Pg and Pg metabolites such as allopregnanolone to the brain would also explain how physiological (10-30 mg) topical Pg might help reduce anxiety, create calmness, and promote sleep as seen with higher dose (100-300 mg) oral progesterone.20 Because topical Pg does not raise serum levels of Pg, very little research effort has focused on percutaneous Pg therapy for benefits of insomnia; however, recognition that the brain glymphatic system might facilitate transport of topically delivered Pg would spur new interest in the use of physiological topical Pg for insomnia.
In men topical T even at high dosage has much less impact on muscle development relative to other forms of T therapy that increase serum levels of T (e.g., im/sc injections, pellets). This is attributed to poor absorption of topical T. However, topical T at pharmacological dosing (50-200 mg) does have a significant impact on brain function as it relates to activation of dopamine receptors and well characterized actions of dopamine to increase aggression, pleasure-seeking activities, and risk-taking behaviors.21
In summary our results presented in Tables 1-3 derived from tens of thousands of female and male patients who have used different doses of topical E2, Pg, and T and tested saliva, capillary blood, serum, and urine metabolite levels of these hormones, reveal that serum and urine provide a less accurate means to monitor topical hormone therapy but are fine for measuring endogenous hormones, or sex hormones delivered by most other methods (e.g., transdermal patch, oral, troche, pellet, or im-sc injection).
Saliva or capillary whole blood (DBS) testing provides a more accurate picture of topical doses needed to achieve physiological levels of these hormones in the capillary beds that are nourishing tissues. Continued use of serum or urine for establishing dosing regimens of topically delivered hormones could, and likely will over time, lead to excessive tissue exposure, which could downregulate (tachyphylaxis) sex hormone receptors and diminish tissue response to the hormone, thereby leading to hormone deficiency symptoms which the hormone therapy was meant to alleviate.
References
- Edelman A, et al. A comparison of blood spot vs plasma analysis of gonadotropin and ovarian steroid hormone levels in reproductive-age women. Fertil Steril. 2007; 88: 1404-1407.
- Du JY, et al. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum, whole blood, saliva, and capillary blood. Menopause. 2013;20:1169-75.
- O’Leary P, et al. Salivary, but not serum or urinary, levels of progesterone are elevated after topical application of progesterone cream to pre- and post-menopausal women. Clin Endocrinol. 2000; 53: 615-620.
- Basdevant A, de Lignieres B. Treatment of menopause by topical administration of oestradiol. In: Percutaneous Absorption of Steroids, Mauvais-Jarvis P, Vickers, CFH., and Wepierre J, Eds., Academic Press (London), 1980; pp. 249-258.
- Prestwood KM, et al. Ultralow-dose micronized 17betaestradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-8.
- Place VA, et al. A double-blind comparative study of Estraderm and Premarin in the amelioration of postmenopausal symptoms. Am J Obstet Gynecol.1985;152:1092-9.
- Powers MS, et al. Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement. Am J Obstet Gynecol. 1985;152:1099-106.
- Chang KJ, et al. Influence of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil Steril. 1995; 63: 785-791.
- Brennan JJ, et al. Serum concentrations of 17betaestradiol and estrone after multiple-dose administration of percutaneous estradiol gel in symptomatic menopausal women. Ther Drug Monit. 2001;23:134-8.
- Padwick ML, Endacott J, Whitehead MI. Efficacy, acceptability, and metabolic effects of transdermal estradiol in the management of postmenopausal women. Am J Obstet Gynecol. 1985;152:1085-91.
- Burry KA, Patton PE, Hermsmeyer K. Percutaneous absorption of progesterone in postmenopausal women treated with transdermal estrogen. Am J Obstet Gynecol. 1999; 180:1504-11.
- Williams Textbook of Endocrinology, Larsen PR, Kronenberg HM, Melmed S, and Polonsky KS, Eds., 10th Ed., Elsevier Health Services (Saunders), Philadelphia, 2003.
- Bhasin S, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678-88.
- Zava D. Testing with Different Body Fluids and the Results of the Hormone Delivery Rate. Anti-Aging Medical News. Amer. Academy of Anti-Aging Medicine. Fall 2013; pp. 47-51.
- Zava DT, Groves MN, Stanczyk FZ. Percutaneous absorption of progesterone. Maturitas. 2014;77:91-2.
- Bhasin S, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-59.
- Leonetti HB, Wilson KJ, Anasti JN. Topical progesterone cream has an antiproliferative effect on estrogen-stimulated endometrium. Fertil Steril. 2003;79:221-2.
- Leonetti HB, et al. Transdermal progesterone cream as an alternative progestin in hormone therapy. Altern Ther Health Med. 2005;11:36-8.
- Skalak TC, Schmid-Schönbein GW, Zweifach BW. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res. 1984;28:95-112.
- Schumacher M, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. 2014;113:6-39.
- Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alphaandrostanediol. Pharmacol Biochem Behav. 2007;86:354-67.
Dr. Zava earned his PhD in biochemistry and has extensive experience researching hormones and breast cancer. He established ZRT Laboratory in 1998 to provide health care practitioners and patients with a deeper understanding of the role hormones play in wellness, and remains a strong advocate of wellness through preventative health. He is the co-author of What Your Doctor May Not Tell You About Breast Cancer and the author of many peer reviewed articles.







