Jonathan E. Prousky, ND, MSc, MA, RP(Qualifying)
Professor, Chief Naturopathic Medical Officer
Canadian College of Naturopathic Medicine
Abstract
This article focuses on what stresses the brain and the different mechanisms that become triggered as a result. The stress-vulnerability model is highlighted to introduce the notion that with sufficient stress there will often be resultant mental health consequences. The concept of allostasis, allostatic load, and allostatic overload are highlighted to demonstrate the non-linear stress mechanisms (i.e., neural, neuroendocrine, and neuroendocrine-immune mechanisms) that help individuals to physiologically adapt to the stresses in their lives. The consequential effects on the body and brain (principally, the prefrontal cortex, amygdala, and hippocampus) that result from allostatic load and allostatic overload are further discussed. Many different sources of chronic stress are noted in reference to brain mechanisms and include the following: prenatal and postnatal early life experiences; social isolation, loneliness, and socioeconomic status; personality factors; medical diseases; and psychiatric illness (mental disorders), and suicide. Since the brain determines how the world is perceived and responded to, everything that chronically stresses this organ is positioned as being of paramount importance to an individual’s current and future morbidity and mortality.
Introduction
Suffering is ubiquitous and inevitable. No living person escapes personal catastrophes, hardships, regrets, failures, loss, disease, and emotional pain. When a person is literally confronted with an overwhelming life situation, at some point – often described as a “breaking point,” or “mental breakdown” – he will seek out assistance from a healthcare professional, such as a family physician, mental health professional, or a psychiatrist. The patient’s psychological distress is usually ascertained to have passed some threshold diagnostically to meet criteria established for having a mental (psychiatric) disorder, or maybe even several mental disorders. No matter what the particular mental disorder or disorders the patient has been diagnosed with, the resultant signs and symptoms of psychological distress happened as a consequence of mediating factors arising from the patient’s stress-vulnerability (or stress-diathesis). The notion of stress-vulnerability was described decades ago in the context of schizophrenia and relapse.1 In simple terms, with sufficient stress combined with significant vulnerability, there will always be some risk of having significant psychological distress resulting in clinically meaningful (and identifiable) psychological signs and symptoms. All patients (and therefore all humans), have their own intrinsic vulnerabilities (e.g., genetics), and when mediated by sufficient psychosocial stressors (e.g., a life crisis, relationship problems, and/or substance abuse), the net result is usually psychological distress often manifested as diagnosable psychiatric illness like major depressive disorder (MDD), generalized anxiety disorder (GAD), and post-traumatic stress disorder (PTSD).
Concepts of Allostasis, Homeostasis, Allostatic Load, and Allostatic Overload
When reviewing contemporary models to explain the aforementioned schemata, certain terms need to be defined before proceeding. Allostasis, coined by Sterling and Eyer,2 refers to biological adjustments that allow an individual to adapt to particular challenges that happen over the lifespan.Adapting to such challenges demands the synchronous though non-linear activation of many different physiological processes, such as neural, neuroendocrine, and neuroendocrine-immune mechanisms.3 Allostasis begins with the brain and happens or is instigated by how an individual perceives and interprets any given situation. Conceptually, however, allostasis is different from homeostasis, which it is often compared to because of related concepts. Homeostasis is about ensuring survival, and refers to “physiological parameters like blood oxygen and pH” that are “maintained within a narrow range” (p.37).3 Allostasis, on the other hand, is about adaptation, but the physiological adaptations may not ensure survival because they can become deleterious over time and cause irreversible damage.

and patient is an important aspect of treatment
and enhances the well-being of both.
Allostatic systems become quickly activated when an individual is confronted by acute stress, which normally return to their baseline states rather quickly. It is more commonly chronic stress, however, that results in problematic sequelae. Chronic stress has been defined as “ongoing demands that threaten to exceed the resources of an individual in areas of life such as family, marriage, parenting, work, health, housing, and finances” (p.638).4 In physiological terms, chronic stress refers to a “pathological state that is caused by prolonged activation of the normal acute physiological stress response, which can wreak havoc on immune, metabolic, and cardiovascular systems” (p.56).5 When an individual is faced with chronic stress, which is common among most psychologically distressed patients, it may seem enduring and without a clear ending. Chronic stress will eventually overwhelm allostasis, and cause allostatic load (AL) and allostatic overload (AO). AL represents body degradation that results from repeated allostatic responses during stressful situations.6 This results when an allostatic system fails to habituate to the recurrence of the same stressor, fails to shut off following overwhelming stress, and/or whose response is deficient resulting in heightened activation of other, normal counter-regulatory systems.3,7 AO is thus an extension of AL, which often results in irreversible damage to body organ systems, and/or mental illness. Thus, unmitigated chronic stress that results in AL and AO will typically cause all sorts of psychological distress signals, especially among individuals vulnerable to mental illness (Table 1).
| Table 1. Psychological Distress Signals of Allostatic Load and Overload (in no particular order of appearance; Adapted from: Prousky8) |
Anxiety and/or persistent or intense episodic panic attacks
Depression
Despair
Helplessness
Insomnia
Lack of optimism (absence of a positive outlook)
Anger
Denial
Guilt
Hostility
Hyperactivity
Isolation
Restlessness
Shame
Addictive behaviors (e.g., cannabis and alcohol) to anesthetize feelings
Inability to delay gratification
Not eating, under-eating, or over-eating
Habitual cutting or the desire to harm oneself
Suicidal thoughts
Homicidal thoughts
Delusions
Dissociation
Depersonalization
Grandiosity
Hallucinations
Hyper-religiosity or bizarre religious beliefs
Mania
Obsessions
Paranoia
Persecutory thoughts
McEwen9 has described the common mechanisms involved in allostatic responses that work in a nonlinear but coordinated manner, such as the autonomic nervous system, hypothalamic-pituitary-adrenal (HPA) axis, and the immune, metabolic, and cardiovascular systems. Activation results in the release of catecholamines from both nerves and the adrenal medulla (i.e., the sympathetic-adrenomedullary system; SAM), and the secretion of corticotropin (a.k.a., adrenocorticotropic hormone; ACTH) from the pituitary, which then results in the release of cortisol from the adrenal cortex.5,9 By contrast, when allostatic responses are attenuated, the aforementioned systems bring cortisol and catecholamines to their baseline levels. This happens when the stressor, or the component that mediated these systems to act, have been contained. AL, on the other hand, happens when the inactivation is insufficient, and then the individual gets exposed to too many stress hormones, which can happen over “weeks, months, or years,” leading to “pathophysiologic consequences” (p.172).9
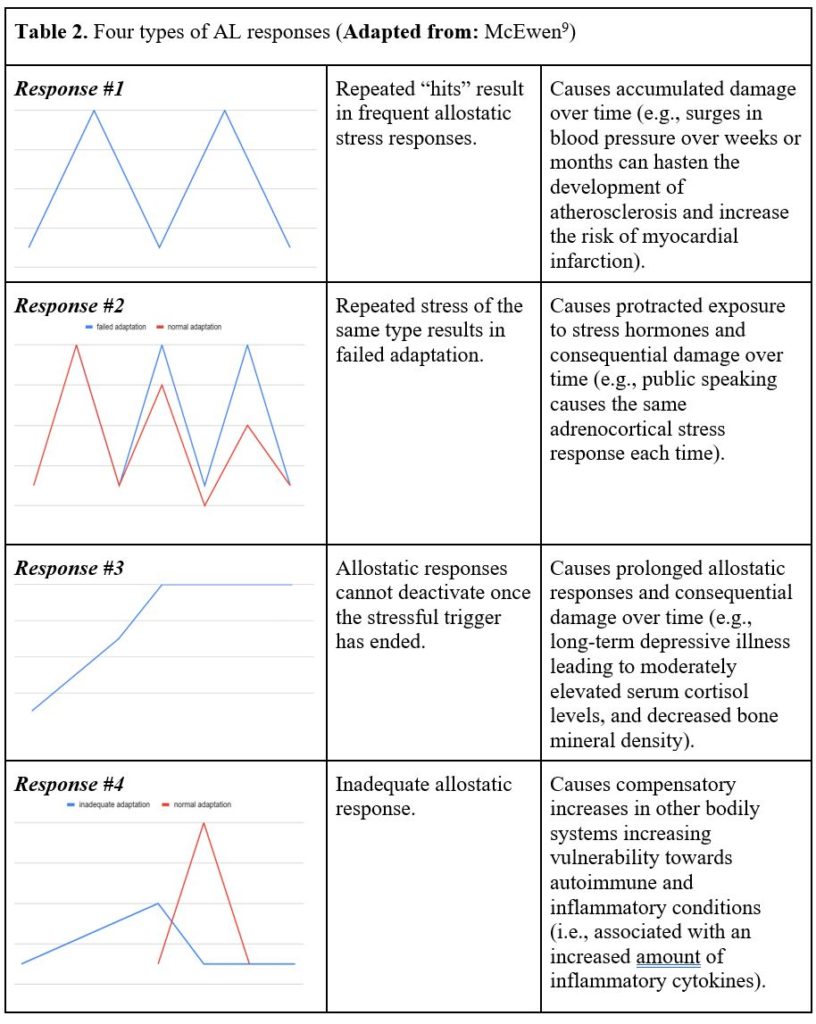
What should be evident and perhaps obvious is that AL not only results in psychological distress, but also in consequences that harm both the brain and body over time. There are apparently four types of responses associated with AL (Table 2).9 The first response involves frequent stress over time, such as surges in blood pressure resulting in myocardial infarction among vulnerable individuals. Or, in primates (which are genetically very similar to humans), repeated elevations in blood pressure over weeks and months can hasten atherosclerosis, and increase the risk for myocardial infarction.
The second response involves being exposed to the same repeated stress, but adaptation is insufficient, which results in protracted exposure to stress hormones.9 An example of this was evidenced in a study in which healthy male subjects were exposed to brief psychosocial stressors (i.e., mental arithmetic and public speaking) in front of an audience.10 The group of men that were denoted as ‘high responders’ displayed large increases in salivary cortisol in response to each of the experimental treatments between days 1 and 5. These men were unable to show any habituation in their adrenocortical stress response despite being exposed to repeated and predictable psychosocial stressors.
The third response happens when the allostatic system cannot be inactivated once the stressful trigger has ended, which then prolongs the allostatic response.9 An example of this has been documented among women with depressive illness, who experienced a long duration of “moderately elevated serum cortisol concentrations” that interfered with the formation of bone, resulting in decreased bone mineral density (p.173).9
In the fourth and final response, the allostatic system does not adequately respond (i.e., under responds), and this results in compensatory increases in other bodily systems.9 For instance, if the secretion of cortisol does not increase when an individual is stressed, there could be a resultant increase in inflammatory cytokines since these substances are normally “counter-regulated by cortisol” (p.173).9 This mechanism is purported to be responsible for the development of autoimmune and inflammatory conditions due to a hyporesponsive HPA axis.
Since both AL and AO operate on a continuum, AO represents a state when organic pathology emerges, which happens when the body breaks down over time due to unmitigated chronic stress and inadequate allostasis. Similarly, AO also emerges when the brain becomes exposed to chronic stress and inadequate allostasis, and its embodiment which is the mind, likewise breaks down and manifests sufficient psychological signs and symptoms characteristic of psychiatric illnesses, such as MDD or GAD.
Specific Brain Regions and Their Associated Stress-Response Mechanisms in General Terms
Specific brain regions – i.e., the hippocampus, amygdala, and prefrontal cortex (PFC) – have been implicated in chronic stress and AL. The hippocampus is a “region in the medial temporal lobe that is instrumental for learning and remembering declarative and spatial information, processing the contextual aspects of emotional events, and regulating visceral functions, including the HPA axis” (p.435).11 The amygdalais anatomically adjacent to the hippocampus, and “rapidly assigns emotional significance to environmental events, and it regulates physiological and behavioral responses to those events” (p.435).11 The PFC is what makes us human and is a large brain region that occupies “the anterior portion of the frontal lobe” is “connected with the hippocampus,” and is “broadly involved in higher cognitive functions (e.g., working memory and executive control), as well as the control of emotion, mood, stress functions, and impulsive actions” (p. 435).11
These brain areas work in a collaborative manner when something is perceived as stressful (i.e., threatening) or even meaningful. This circuitry involves the amygdala and hippocampus – i.e., limbic brain structures – “that process experiences by interfacing with lower vegetative brain areas (such as the hypothalamus and brainstem) and higher cortical areas, particularly the prefrontal cortex” (p.434).11 So there are top-down mechanisms whereby the PFC attempts to exert some measure of control over limbic brain structures, and there are bottom-up mechanisms whereby limbic brain structures exert some measure of control over the PFC. Of particular importance is that the projections from the amygdala to the PFC are greater than those projections coming from the PFC to the amygdala.12 As a result, our emotions can sometimes take over and control behavior because our amygdala, when triggered by fear, exerts more influence over how the PFC operates.12 As one well respected researcher noted, “this hostile takeover of consciousness by emotion” happens because emotions “monopolize consciousness, at least in the domain of fear, when the amygdala comes to dominate working memory” (p.226).13
As we just noted, fear is an important mediator or perhaps mobilizer of activity, and yields physiological and behavioral consequences – such as fight, flight, or freeze – that have been experienced by all human beings. This brings us to another part of our brain’s limbic system, called the thalamus, which functions as a central relay station by sending motor and sensory signals to the cerebral cortex (i.e., houses the PFC) to be processed and interpreted.12 So when distilling the pathways involved in strong emotions, such as fear, there are two pathways that ought to be reviewed further since they have important treatment implications, which will be addressed in future articles. The first pathway involves sensory information proceeding from the thalamus to the cerebral cortex (and PFC), and then to the amygdala, which then activates physiological responses that includes the sympathetic nervous system (SNS) and hormones such as cortisol and epinephrine (a.k.a., adrenaline) to assist our biology in getting mobilized for action.12 The second pathway involves sensory information proceeding from the thalamus directly to the amygdala (i.e., as a result of being triggered by experiences that previously created fear), initially bypassing the cerebral cortex (and PFC), and directly activating the SNS as described above.12
With respect to the hippocampus and how it interfaces with the above-mentioned brain pathways, there is a lot of cross-talk that happens between the hippocampus and amygdala, and between the hippocampus and PFC. For example, should any of the aforementioned pathways become activated, the hippocampus (i.e., is rich in glucocorticoid receptors) can modulate the stress response by inhibiting or activating corticotropin-releasing hormone (CRH) from the hypothalamus, which plays an integral role in the eventual release of cortisol from the adrenal cortex.14 In situations of chronic stress, the hippocampus can become impaired or disrupted, and won’t be able to terminate the stress response, leading to heightened HPA activity, and a consequential augmentation of damage-inducing adrenal steroids over the long-term.11 Moreover, the hippocampus receives information from the PFC (just like the amygdala), and also influences the PFC regarding the importance of external stimuli, including threatening information (e.g., fear).14 The hippocampal stress response is also linked to the amygdala’s stress reactivity, but it differs in the way emotional events become managed. Whereas the amygdala tags emotional content, such as fear, the hippocampus further tags emotional content by forming episodic representations of emotional content in terms of its contextual meaning.15
All of this information has relevance to the pathways described earlier because when something is deemed chronically stressful by context-driven emotional experience, for which there was an initial strong reaction by the amygdala, the hippocampus undergoes specific neuroplastic changes that result in diminished coupling with the PFC. This results in increased stress-vulnerability to life experiences and consequently less top-down control.15 Stated another way, diminished hippocampal functionality impairs the PFC’s inhibitory control over the amygdala.16 Diminished hippocampal functionality from chronic stress will further undermine an “individual’s ability to process information in new situations and to make decisions about how to deal with new challenges or stressors” (p.435).11
To summarize the information presented above on brain structures and stress-related mechanisms, it can be ascertained that (1) the amygdala plays a prominent role in emotional processing; and (2) these three brain structures interface with each other and “unite aspects of cognition, memory, executive function with elements of emotional regulation” (p. 1170).16
Stress, Atrophy, and Damage to Specific Brain Structures
With chronic stress and AL, the brain undergoes plastic changes, which results in atrophy of the hippocampus, amygdala and PFC.17 While chronic stress does lead to structural plastic changes, the human brain does possess “a life-long and clinically significant capacity for reversible, structural plasticity” (p. S22).7 This is good news since the effects of chronic stress can at least, in part, be attenuated by appropriate measures taken by an individual over the course of his lifetime.
With chronic stress, dendrites in neurons in the hippocampus and PFC shrink, become shorter and less branched, and these changes result in diminished synaptic output.7 These changes further compromise an individual’s “capabilities for nuanced cognitive function, memory and self-regulation” (p.S22).7 On the other hand, the same type of chronic stress causes an expansion of dendrites and increased synaptic input to an area of the amygdala known as the basolateral amygdala, which results in heightened anxiety, aggressiveness, and vigilance.7
One hypothesis that has been advanced is the glucocorticoid cascade hypothesis (GCH) of stress and aging, which refers to the chronic inability of hippocampus to shut off the HPA axis, which leads to persistent damage to this brain structure and PFC over time.3,7 Glucocorticoids also potentiate the release of damaging extracellular levels of excitotoxic amino acids (EAA) under stress, such as glutamate, and this happens within the hippocampus, and other brain regions.17 Glial cell depletion (or alterations) have also been implicated in atrophy of brain regions like the hippocampus, amygdala and PFC.17 The fact that all of these particular brain areas become targets of chronic stress suggests that a common mechanism may underlie the resultant atrophy and damage that has been noted.17
Of interest is the notion that glucose availability within the brain plays a role in mediating resilience or damage. A deficit or lack of available brain glucose can mechanistically lead to excitotoxic cell death within the hippocampusand likely other brain areas, whereas sufficient brain glucose may reduce excitotoxic cell damage.17,18 A lack of available glucose within the brain has even been proposed as a limiting factor in an individual’s free will because self-control demands available brain glucose, and with less available brain glucose, behavior (and therefore self-control) become more limited until brain glucose levels are repleted or restored to normal.19 Though there are other mechanisms (i.e., such as an evolving number of genomic-mediated molecules implicated in stress-induced dendritic remodeling20) to account for neuronal damage, glucocorticoids, brain glucose debt or insufficiency, an unrestrained release of EAA, and glial cell depletion (or alterations) cause neuronal death and therefore neuronal loss in these particular brain areas.
Prenatal and Postnatal Early Life Experiences Alter Biology and Create Life-Long Vulnerability
Research has consistently shown that early life experiences promote a more vulnerable or hardy (i.e., resilient) human being. McEwen and Getz7 reviewed animal research demonstrating that “early life experiences become biologically inscribed” (p.S22). They noted that factors before birth (e.g., prenatal stress) and those occurring postnatally (e.g., extended separation of the infant from mother) can impair normal brain development and function.By contrast, adequate maternal care and consistent caregiving benefited the offspring by creating durable patterns of reduced anxiety, more efficient stress reactivity, and better social and cognitive development. There are also transgenerational effects that have been shown to be behaviorally and genetically transmitted by mother rats to their offspring. For instance, environmental manipulations that alter maternal rat behavior will impart nongenomic differences in stress reactivity across generations of female offspring.21 Similarly, environmental manipulations that increase maternal stress during pregnancy resulted in genetically transmitted alterations in HPA axis reactivity to stress and anxiety-like behaviors among second generation male rats.22
In humans, similar findings have shown that stress does become biologically inscribed in human fetuses and children and yields effects that endure well into adulthood. One of the more salient (and perhaps extreme) examples of this comes from an extensive systematic review done on the intergenerational (or transgenerational) effects of Holocaust survivors and the mental health of their offspring (a.k.a., Holocaust survivor offspring; HSO).23 The findings of this review yielded convincing evidence of intergenerational effects that showed associations between parental mental health, perceived parenting and attachment quality, and increased psychiatric symptoms among HSO, including high conflict and less cohesion within families of HSO. Having two survivor parents was associated with greater mental health problems among the HSO compared to having only one survivor parent. The HSO also exhibited a heightened vulnerability for stress, but this seemed to only happen when faced with genuine danger. Lastly, intergenerational effects on cortisol modulation (i.e., levels) was also evidenced among the HSO.
Heritable factors, such as genetics, also represent an avenue that exposes how stress becomes biologically inscribed. For instance, some human carriers of a particular allele—i.e., the methionine allele of the valine 66met brain-derived neurotrophic factor (BDNF) polymorphism or the Val66Met polymorphism in the BDNF gene—have inherited genetics that influence the expression of this important growth factor.11 BDNF is a “major neurotrophic factor that plays an important role in the formation, guidance, and survival of neurons during development but also in synaptic plasticity and survival in the adult brain” (p.410).24 Having this polymorphism can result in lower grey matter volume in the hippocampus and PFC due to alterations in the production and expression of BDNF, thus, undermining synaptic or cellular plasticity and neurogenesis in response to stress exposure.11 This particular polymorphism is also linked to impaired episodic memory, as well as cognitive impairment in older adults more than 55 years of age.25
In keeping with our discussion about genetics, other alleles can impact stress regulation over the course of a person’s life. Aggression from difficulties coping with stress, for example, can arise due to genetic variants that are associated with increased monoamine oxidase-A activity (i.e., results in an increased breakdown of monoamine neurotransmitters, such as dopamine, norepinephrine, and serotonin).26,27 This speaks to the concept known as reactive alleles, which refers to how some gene variants increase or decrease in response to environmental influences like stress.7 The point is that all allelic variants are in a sense reactive, whether the heritable variants involve BDNF or those variants associated with monoamine oxidase-A—for when in combination with environmental stressors—phenotypic expression changes, which may facilitate allostasis, or may result in enduring patterns of AL and/or AO.
The length of telomeres is another example of how stress becomes biologically inscribed. A telomere is a region situated at each end of a chromosome, and which protects the chromosome from deterioration while preserving vital genetic information.28 Age-related health problems and diseases have been consistently associated with excessive or accelerated telomere shortening.28 Stress, resulting in the release of glucocorticoids, has been shown to reduce the levels of antioxidant proteins, leading to increased oxidative damage to DNA, and quickened telomere shortening.28 Cross-sectional human studies have demonstrated associations between compromised telomere integrity and high levels of psychosocial stress exposure.29 Even factors that happen before birth, such as prenatal stress exposure, have been shown to be a significant predictor of subsequent shorter leukocyte telomere length in young adulthood. These findings are believed to represent an important biological pathway that broadly influences the “developmental origins of adult health and disease risk” (p.E513).29
Other, and perhaps more relevant research to the practicing clinician, involves associations between large cohorts (i.e., involving several thousand individuals or more) that experienced adverse childhood experiences (known as ACEs) and enduring problems in adulthood. ACEs have been described by the CDC as potentially traumatic experiences happening in childhood (i.e., between 0-17 years of age) that comprise a child’s sense of safety, security, and bonding, and include all or some of the following: witnessing or experiencing violence in the home or community; experiencing neglect; having a family member attempt or die by suicide; and being in a household with substance misuse, mental health problems, or parental separation or members of the household being in jail or prison.30 Increasing amounts of ACEs have been associated with adult health risk behaviors and diseases.31 Compared to individuals without any ACEs, those exposed to four or more categories of ACEs experienced a four- to 12-fold increase in alcoholism, drug abuse, depression, and suicide attempt. The same individuals had a two- to four-fold increase in smoking, poor self-rated health, 50 or more sexual intercourse partners, and sexually transmitted disease. There was also a 1.4- to 1.6-fold increase in physical inactivity and obesity. This data was sadly presumed to have underestimated the actual consequences of ACEs, and their relationship to adult risk behaviors and diseases.
Additional data has demonstrated that a graded relationship exists between ACEs and the risk of attempted suicide.32 Merely having experienced an ACE was associated with a two- to five-fold increased risk of attempted suicide. Individuals without ACEs were shown to have a 1.1% prevalence of attempted suicide. Though the statistical marker known as odds ratio is somewhat different from prevalence, individuals with seven or more ACEs had an odds ratio of attempted suicide of 31.1%. Other studies evaluating the impact of ACEs demonstrated an association between elevated ACE exposures or scores and problems in adulthood that include childhood autobiographical memory disturbance,33 alcoholism and depression,34 depressive disorders,35 and hallucinations.36
There are also consequential changes to brain structures and function, and overall physiology that result from ACEs. Several examples are provided here to demonstrate this association. A report that documented a 10-year history of children growing up with mothers having chronic depression, showed larger amygdala volumes in the childrens’ brains.37 Another report identified increased negative functional connectivity between the PFC and amygdala among adolescents exposed to physical, sexual, or emotional abuse compared to adolescents without a history of maltreatment.38 These findings were more pronounced among adolescents that had more severe abuse, and were also associated with higher amounts of externalizing psychopathology (e.g., opposition/conduct and attention) disorders) some two years later. Other data has shown that children raised without sufficient verbal stimulation, and in unstable home environments were more likely to develop impaired cognitive function, increased systemic inflammation, cardiovascular disease, substance abuse, anti-social behavior, and depression.39,40
Above all, prenatal and postnatal stress does become biologically inscribed by adversely impacting vulnerable brain structures, altering physiology, and shaping adult development. Not surprisingly and almost in a rather banal way, a combination of these aforementioned factors disrupt allostatic mechanisms to such a great extent, practically guaranteeing AL and AO, psychopathology, physical disease, and a cascading path of enduring mental and physical problems lasting well into adulthood.
Chronic Stress and Social Isolation, Loneliness, and Socioeconomic Status
Similar to prenatal and postnatal stress, social isolation, loneliness, and socioeconomic status (SES) activate allostatic systems, and result in AL and AO due to detrimental life outcomes, pathophysiological changes, and consequential brain changes.
Social isolation and loneliness are common experiences that all of us have endured (or will endure) at certain times over the course of our lives. In a meta-analytic review on loneliness and social isolation, Holt-Lunstad et al defined these terms and then analyzed aggregated data to determine associated health outcomes.41 Social isolation was defined as living alone, having infrequent social contacts, and having sparse social network connections. Loneliness represented the subjective experience of social isolation, resulting from disparities between one’s desire for social relationships and one’s actual social relationships. The results of the meta-analytic review showed social isolation and loneliness to yield weighted average effect sizes that increase the risk of mortality in a manner comparable to other health risk factors like obesity, substance abuse, physical inactivity, and mental health problems. Specifically, the odds ratio of increased mortality was 1.29 (29%) for social isolation, 1.26 (26%) for loneliness, and 1.32 (32%) for living alone.
In a review paper, Cacioppo and Hawkley described underlying mechanisms associated with social isolation.42 Individuals that perceive themselves to be socially isolated often experience insecure adult attachments that beget physiological changes characterized by activation of the sympathetic nervous system (SNS), the SAM system, and the HPA axis. Additionally, perceived social isolation becomes its own stressor and produces negative emotions (e.g., depression and anxiety), negative reactivity (e.g., hostility, mistrust, and irritability), and reduced feelings of self-worth, happiness, and life satisfaction. The autonomic patterning of socially isolated individuals is also characterized by a higher total peripheral resistance, and lower cardiac output. These latter effects degrade both central and peripheral hemodynamics leading to increased vascular resistance and decreased vascular compliance, and the likely development of hypertension. Perceived social isolation also undermines repair and maintenance functions and weakens anabolic processes, such as wound healing time and restorative sleep (i.e., lower sleep efficiency and higher wake times after sleep onset).
Similar to social isolation and loneliness, SES is also a significant stressor since it imposes disadvantages and impediments that undermine a person’s ability to succeed in life for countless reasons. McEwen and Getz cited several sources when documenting the deleterious effects of low SES and perceived SES.7 They cited research demonstrating that individuals having low SES are at greater risk for “predisease” conditions, such as obesity, metabolic syndrome, substance abuse and psychiatric disorders (p.S23). Similarly, they noted other research that showed an association between perceived low SES and a poor sense of control and low self-esteem.
With respect to brain changes, there is evidence of deleterious effects arising from social isolation, loneliness, and SES. For example, the chronic stress associated with individuals perceiving themselves to have a low social standing (i.e., a composite marker that includes standard measures of SES) was linked to reduced grey matter volume in the anterior cingulate portion of the PFC.43 This was deemed important because this particular neuroanatomical area plays a role in how people experience emotions and regulate their behavioral and physiological reactivity to psychosocial stress. Even low perceived parental standing—known to be a likely indicator of socioeconomic hardship during childhood and adolescence—was “associated with greater amygdala reactivity to threatening (angry) facial expressions” in “healthy individuals who had not yet reached their adult SES” (p.203).44 This finding was believed to represent a neurobiological pathway by which early SES experiences become biologically embedded, impacting allostatic systems, and likely future health and disease vulnerability.44
Research on social isolation has reviewed brain changes among individuals that spent 14 months living in isolation in the Antarctic.45 The results showed statistically significant reductions compared to controls in the hippocampal volume of the dentate gyrus from before to after the expedition. This particular part of the hippocampus contributes to the formation of episodic memories.46 The results showed that other hippocampal regions and even several regions of the PFC had reduced volumes compared to controls but these changes did not reach statistical significance.45 Serum BDNF levels were also measured before and after the expedition. Compared to serum measurements before, BDNF levels dropped during the expedition and did not recover to their pre-expedition levels when assessed 1.5 months after the expedition had ended. The decreased serum BDNF levels that happened during the expedition were also associated with reductions in the hippocampal volume of the dentate gyrus, and reduced cognitive performance (i.e., as shown by tests of spatial processing and selective attention). The results of this study demonstrated how vulnerable the dentate gyrus of the hippocampus is to environmental deprivation, and revealed similarities to animal studies in which “neurogenesis, stress-induced behavioral changes, and environmental deprivation” adversely impacted this particular hippocampal region as well (pp.2274-2275). Even though the sample size was very small in this study (n=9) and other factors might have contributed to the observed brain changes from environmental deprivation, it is clinically plausible that similar brain changes and reductions in BDNF and cognitive performance will be found among people living socially isolated lives.
Chronic Stress and Personality
It would seem important to consider personality as a potential influencer of chronic stress, as it plays an essential role in facilitating and/or moderating how the brain and body respond to ongoing challenges. Canli extensively reviewed extraversion (E) and neuroticism (N)—both of which are heritable personality traits—and determined associated brain imaging mechanisms.47 Neuroticism as a personality trait encompasses individuals that are more likely to be moody and to experience feelings such as worry, fear, anxiety, anger, frustration, guilt, depressed mood, and loneliness. Extroversion as a personality trait refers to individuals that are sociable, talkative, assertive, excitable, have lots of energy, and tend to be full of life and energy. Canli’s study showed that Individuals with dominant E traits experience more positive affect in their daily lives compared to individuals with dominant N traits that experience more negative affect in their daily lives. These personality orientations were enduring and noted to last “for periods of up to 10 years” (p.1106). The affective associations between individuals with E and N personality dispositions may also be mediated to some extent “by cognitive biases in the processing of emotional stimuli” (p.1107).47
When Canli evaluated subjects with brain imaging, he found differences between these two types of personality dispositions.47 Individuals high in E showed greater activation of the amygdala when positive images or happy faces were shown. Individuals high in N exhibited greater activation of the amygdala when negative images were shown. Why does this seem important? The amygdalar activation was oriented towards positive and negative stimuli and was specific to these personality types. These brain differences in personality were also postulated to have some involvement in resilience and vulnerability factors for specific types of psychopathologies. Cited data pertaining to individuals high in N (or higher N relative to lower E) showed more vulnerability towards the development of eating disorders, low self-esteem, post-stroke depression, and more unfavorable outcomes when being treated for depression.
Interested readers may want to additionally review an important cohort study that documented increased all-cause mortality among individuals having a N personality disposition (i.e., based on a composite of pessimistic, anxious, and depressive personality traits).48 These individuals were assessed early in life, and their N personality disposition had significant negative consequences on mortality when evaluated over four decades (i.e. a hazard ratio of 1.42 or 42% in the primary analysis).Other studies were also cited showing a relationship between N personality traits and increased mortality (see Table 5, p.498).48 Some of the biological mechanisms believed to be responsible for the increased all-cause mortality among individuals with a dominant N personality disposition included the following: hippocampal atrophy and other brain lesions; stress and depression causing “increased heart rate, hypertension, increased plasma norepinephrine levels, or changes in blood coagulation that may increase the risk of cardiovascular disease”; and stress causing reduced immune system responsiveness and a greater risk of cancer (p.496). Other mechanisms attributed to individuals with the N personality disposition and the finding of increased all-cause mortality included poor self-care, unhealthy behaviors, and underutilizing healthcare resources and/or poor compliance with treatment.
There are certainly other personality types—i.e., other than N and E—that modulate chronic stress, impact the brain, and are also associated with diverse health outcomes. The N personality type was highlighted here because it seems to more commonly occur in my clinical practice and is perhaps more deleterious than other personality types in relation to chronic stress and adverse health outcomes.
Chronic Stress and Medical Disease
As mentioned near the beginning of this paper, there are four different types of allostatic responses, and for three of them the resulting biological adaptations would be required to manage alterations in cortisol9 (as well as adaptations resulting from activation of the SAM49) over prolonged periods of time.In this section, I will briefly review the links between chronic stress and medical (i.e., physical) disease, and highlight what happens when the body is unable to cope with stress resulting in a breakdown of bodily resources. For example, chronically elevated cortisol levels due to persistent or poorly managed psychosocial stress could promote insulin resistance leading to weight gain and obesity. In one such publication, the dichotomous roles of cortisol were described in relation to what it does in the short-term compared to its deleterious effects when there is persistent and poorly managed psychosocial stress over the long-term.50 When the physiological stress response leads to the release of cortisol, there will be an abrupt impairment of insulin secretion and an increase in the production of hepatic glucose. However, should the stress response persist for a prolonged duration, it will inhibit insulin secretion, impair insulin-mediated glucose uptake, and disrupt insulin signaling within skeletal muscle. Individuals can compensate homeostatically by increasing pancreatic beta-cell function, or by increasing the release of insulin. However, when stress becomes prolonged, insulin resistance develops, or becomes worsened by established obesity, resulting in biological adaptations that cause hyperglycemia and adverse metabolic consequences.
In addition to weight gain, obesity, and metabolic consequences, there are other stress-related diseases that are presumed to result from prolonged biological adaptations to cortisol secretion, including biological adaptations resulting from activation of the sympathetic-adrenal-medullary system. The list includes asthma, gastrointestinal diseases (e.g., ulcerative colitis), functional gastrointestinal diseases, coronary heart disease, rheumatoid arthritis, migraine headaches, being more susceptible to cold viruses, nonalcoholic liver disease, neurodegenerative disorders (e.g., Alzheimer’s and Parkinson’s disease), and even cancer.51,52 The list of diseases that are stress-related, however, is likely to be much greater since prolonged (i.e., chronic) stress is considered a common risk factor in 75%-90% of all diseases.52
The implicating factor in all these diseases involves an inability to discharge or effectively manage perceived stress over time, which of course strongly implicates the brain because it is the primary organ that mediates how a person perceives and responds to chronic stressors. In fact, the continued uncertainty (i.e., when anticipated outcomes are unexpected) associated with chronic stress is believed to create a situation in which the brain cannot meet its demand for extra energy, and because of failed stress habituation (i.e., the inability to “attenuate autonomic, endocrine, and metabolic reactions when repeatedly exposed to the same hostile environment”), the resulting AL and subsequent AO yields both systemic pathology (as described above), as well as brain malfunction or pathology (will be described in the next section; p.167).53
Chronic Stress, Psychiatric Illness (Mental Disorders), Suicide, and Brain Mechanisms in General Terms
When faced with chronic stress, which seems to always be adjoined with the subjective experience of uncertainty, some individuals will be able to habituate (a.k.a., habituators) and attenuate their biological stress mechanisms. However, some individuals will be unable to habituate, and therefore their biological stress mechanisms will continue to disrupt the healthy functioning of their entire biological system (i.e., resulting in AL), which adversely impacts both the brain and body. As pointed out by Peters et al, when uncertainty cannot be resolved this leads to a “vicious cycle of altered brain architecture and systemic pathophysiology, which further damages the capability of the subject to cope with uncertainty” (p.168).53 When this proceeds for too long or when resources to attenuate the chronic stress become depleted and damaged, AO ensues, and gives rise to brain malfunction or pathology.53
It is of no surprise then that life events, such as the unexpected COVID-19 pandemic, are major players in the difficult subjective experience of uncertainty. The brain must have sufficient energy to meet its high metabolic demands in the face of chronic stress and uncertainty. When there is failed habituation, which happens for some individuals, the levels of self-esteem and locus of control are significantly diminished, placing the individual at high risk of mental morbidity and mortality.53 With respect to mental morbidity, there also exists a significant body of literature demonstrating a relationship between life events and the onset of psychiatric illness. Apparently, the strength of associations between stressful life events (i.e., also often mired in uncertainty) and psychiatric illness is stronger than similar associations and the development of medical disease.51
Though the names of psychiatric illnesses differ diagnostically, what is apparent is that the brain mechanisms implicated in chronic stress are more similar than dissimilar across a broad range of neuropsychiatric phenomena. For example, when an individual is faced with uncertainty arising from chronic stress and cannot habituate, specific areas of the PFC activate the anterior cingulate cortex (ACC) because it “assesses the degree of uncertainty about whether future outcomes are uncertain”(p.166).53 This results in activation of the amygdala and an ensuing stress response – mediated by the release of norepinephrine – that leads to a hypervigilant state, and simultaneous activation of the SNS (increases glucose for energy utilization) and the HPA axis (increases cortisol) that plays a vital role in synaptic plasticity and learning after stress.53 The released cortisol passes through the blood-brain-barrier and binds to glucocorticoid receptors in and on neurons of the amygdala, hippocampus, and PFC (i.e., three key brain structures mentioned earlier in this paper).51 In simple terms, the net result leads to feelings of threat and loss of control, concomitant with damaging alterations to brain architecture within these three key brain areas, and greater bottom-up control via the ACC-amygdala complex that is not being properly attenuated by specific areas within the PFC.53
When looking at a broad range of psychiatric illnesses, it should come as no surprise that the many of the same brain mechanisms are implicated. This strongly suggests that chronic stress is a significant or major underlying trigger for the majority of psychiatric illnesses. As Patriquin and Mathew pointed out, “Chronic stress may be one cross-cutting construct or dimension (i.e., that occurs across diagnostic categories defined by the DSM-5) related to GAD, as well as other diagnoses (such as highly related MDD)” (p.2).54 In the section below, I will review brain mechanisms in general terms that become triggered by chronic stress for several common psychiatric illnesses, such as GAD, MDD, PTSD, and even borderline personality disorder (BPD).
In GAD, for example, the ACC-amygdala complex gets activated under situations of chronic stress, resulting in decreased connectivity between the amygdala and PFC, making it very difficult for such individuals to effectively regulate their emotions.54 These brain circuit issues are implicated in symptoms, such as anxious arousal, attentional bias to threat, avoidance, and even helplessness behavior.54 Similarly, in MDD, chronic stress leads to impairment in PFC function, an overactivated amygdala resulting in more fear-based or bottom-up control, and a concomitant downgrading of hippocampal functioning.55 Some of the common features of MDD, such as neurocognitive impairment, withdrawing from aversive environments, and anhedonia are linked to these brain circuit issues.55 In PTSD, there is impaired PFC function, elevated cortico-amygdala activity resulting in heightened vigilance and reactions to threatening stimuli, and reduced cortico-basal ganglia reward sensitivity.56 In an older publication on PTSD, the amygdala was noted to exert an overarching influence upon the hippocampus causing reduced functionality and hypermnesia for stressful experiences, behavioral disinhibition, and impaired PFC function.57 In BPD, affect dysregulation happens because certain areas within the PFC are known to be impaired (i.e., as evidenced by abnormal neuronal activity and prefrontal hypometabolism), and is also associated with top-down processing problems.58 Disruptions in prefrontal-limbic circuitry have also been found among people with BPD due to functional disconnectivity between the amygdala and various regions of the PFC with associated volume reductions noted in the amygdala, hippocampus, and in several other subcortical brain regions.58 The amygdala is also believed to be overactive or poorly controlled among BDP patients, such that it directs “their unfiltered attention to predominantly negative and threatening social stimuli” (p.842).58
Outside of psychiatric illnesses, suicide has not specifically been addressed though chronic stress and vulnerability have been proposed as the key triggers toward dying by suicide. Heeringen and Mann noted that “suicide is the result of an interaction between state-dependent (environmental) stressors and a trait-like diathesis or susceptibility to suicidal behavior, independent of psychiatric disorders” (p.63).59 For a brief review of additional theoretical models of suicide see the reference noted here.60 Similar to our previous discussion on childhood adverse experiences, early life adversity appears to affect suicide risk because of alterations to brain architecture, dysregulation of the stress response, and likely cytotoxic effects from excessive concentrations of increased CRH and glucocorticoids.59 In terms of neurobiological mechanisms, certain genetic variants (i.e., alleles for lower expression of the serotonin transporter gene 5HTTLPR) associated with suicide are linked to “impaired connectivity between the prefrontal cortex, amygdala, and anterior cingulate” (p.68).59
Though the clinical manifestations of the aforementioned psychiatric illnesses are both similar and different, they are also principally related to the same key brain areas. Suicide and certain genetic variants were also noted to involve the PFC, amygdala, as well as other brain areas. The genetics and epigenetics, however, of the noted psychiatric illnesses (as well as others) and suicide is very complex and plays an important role in how the brain mediates stress, and the ensuing biological responses. Interested readers should review the following references(i.e., 54-59, 61) to further understand this complex subject matter.
Conclusion
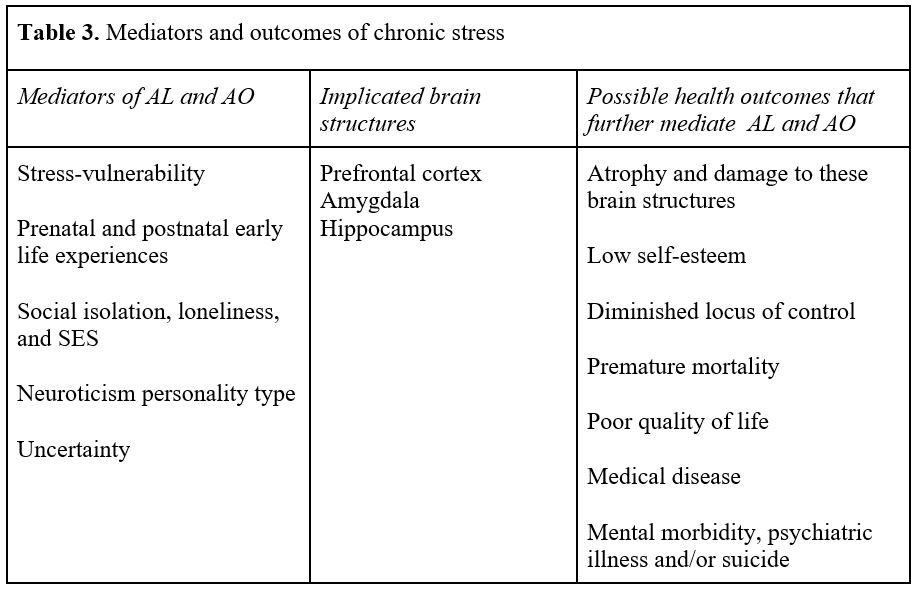
There ought to be no further dispute or controversy about the devastation that chronic stress imposes on both the body and brain. Chronic stress, as noted earlier, is pathological and happens when allostatic mechanisms fail to adapt because resources have been exhausted. The brain, the principal organ that perceives and determines how an individual responds to the world, is subject to multiple stressful and chronic insults that increase the risks of body and brain damage, disease, psychiatric illness, and other health outcomes (Table 3). Thus, understanding what stresses the brain is of paramount importance for any clinician whose aim is to provide treatment that regulates the brain and alters a patient’s current and future morbidity and mortality.