by Michael Ash, BSc, DO, ND, DipION; Robert Settenari, MS; and Prof. Garth L. Nicolson, PhD
Overview
At some point we all experience fatigue, but it usually resolves on its own and is easily explained. Sometimes it has a straightforward organic explanation, sometimes not. For many, however, such organic explanations fail to present a validated route to satisfactory resolution. As research into persistent fatigue has progressed, most clinicians comprehend that all explanatory models of the causes and mechanisms of fatigue and exhaustion proceed from the assumption that they are complex, multifactorial processes. For example, fatigue has been proposed to have biochemical, immunological, emotional, behavioral, and cognitive components, to name a few that contribute to overall fatigue.
Fatigue is usually understood as a loss of overall energy and inability to perform even simple tasks without exertion. At the cellular level, fatigue is related to adversely altered cellular energy systems found primarily in the cellular mitochondria.
Fatigue is the most common complaint of patients seeking general medical care; between 7% and 45% of primary-care consultations involve fatigue as a major complaint.2 It appears that some degree of fatigue may be identified in nearly all of the population, indicating that fatigue is not simply an individual problem; it is also a public health problem.3 Fatigue can progress to the point that it causes disability comparable to that found in chronic medical patients.4,5
At the biochemical level, fatigue is related to the metabolic energy available to tissues and cells, mainly through mitochondrial electron transport. Electron transport is directly linked to functional, intact inner mitochondrial membranes. Thus the integrity of mitochondrial membranes is critical to cell function and energy metabolism.1
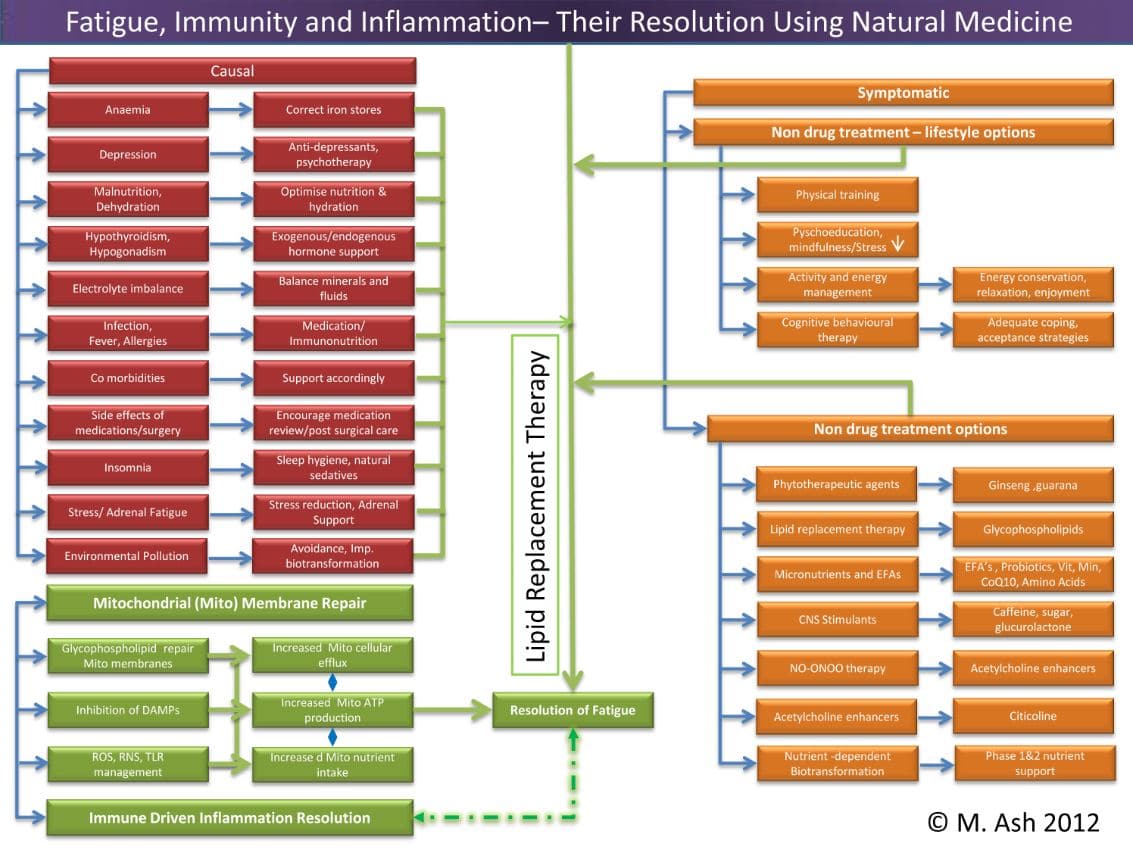
Mechanisms of Fatigue and its Resolution
The successful management or resolution of fatigue is also important in various physical activities of relatively healthy men and women, such as work and sports performance.
Many who experience fatigue do not initially seek primary care intervention but self-treat with stimulants such as central nervous system agonists that include caffeine, herbs, and sugars. Although these provide short-term increases in energy and perception of reduce fatigue, they have potential long-term adverse health effects.6,7
Fatigue: Successful Intervention
Recent clinical trials using patients with chronic fatigue have shown the benefit of an oral non– central nervous system agonist: lipid replacement therapy (LRT).8 Using naturally occurring glycophospholipids, cofactor nutrients, and probiotics, mitochondrial electron transport function can been restored. The result is that moderate to severe chronic fatigue can be significantly reduced in the process.9,10
Additional studies have also identified significant improvements in energy, mood, affect, and function in a healthy population following ingestion of the glycophospholipids and cofactor nutrients in a drink.11,12 These additional natural agents (principally antioxidants and bacterial mediators of immune tolerance) have also shown benefits from a molecular perspective in the inhibition of immune mediated inflammation and associated fatigue.13-16
In recent studies using LRT and cofactor nutrients for 6 months or more has also reduced the blood levels of the amino acid homocysteine, which are related to increased risk for cardiovascular disease, stroke, cognitive loss, depression, and immune dysfunction.17
The role of glycophospholipids and other food-derived agents are attractive as safe and effective interventions in the treatment of persistent and transient fatigue. Studies have been conducted on various populations from those with normal health and function to those undergoing complex treatments for cancer and those with persistent fatigue. These groups have shown between 30% to 40% improvement in fatigue perception and function utilising the internationally recognised Piper Fatigue Scale.18,12
LRT is a dietary approach to replace damaged cellular lipids with undamaged (unoxidised) lipids to ensure proper function of cellular structures, such as cellular and organelle (mitochondrial) membranes. LRT can result in the cellular delivery of unoxidised, undamaged membrane glycophospholipids in order to replace damaged lipids and restore function to cellular membranes. LRT has proven to be an effective method to prevent ROS/RNS-associated changes in function and for use in the treatment of various clinical conditions.
Mitochondria are responsible for many metabolic circuits and signalling pathways. Just a few examples of these are oxidative phosphorylation, the mechanism our cells use to generate most intracellular ATP (cellular fuel); biosynthesis of key molecules, including heme and certain steroids, as well as in many catabolic–energy relevant pathways such as the β-oxidation of fatty acids; and regulation of calcium homeostasis. Importantly, mitochondria are responsible for production of most of our cell’s reactive oxygen species (ROS) and some reactive nitrogen species (RNS). Significant oxidative damage to mitochondrial membranes also represents the point of no return of programmed cell death pathways that culminate in apoptosis or regulated necrosis.19
Immune Inflammation
Of clinical interest from an immunological perspective, recent studies suggest that mitochondria are significant players in the orchestration of innate immune responses via activation of a multiprotein complex called the inflammasome which results in the production and release of the pro-inflammatory cytokines IL-1β and IL-18.20,21
These, in turn, contribute to defensive and coordinated management of the bacterial organisms that reside in our digestive tract. The digestive tract is home to trillions of bacteria and this represents the site of greatest density of innate immune receptors in the body. These receptors are key mediators in the management and maintenance of immune response and tolerance. Their inappropriate activation and expression by IL-1β and IL-18 leads to altered innate immune hyperresponsiveness and may contribute to immune mediated inflammatory diseases as well as fatigue through the subsequent development of persistent molecular inflammation.
Damage to mitochondrial components, especially the delicate inner mitochondrial membrane, leads to the cytosolic release of toxic proteins (caspases and noncaspases) that are normally confined in the mitochondria. These released proteins then bind to specialised innate immune inflammasome activating receptors called nucleotide-binding-and-oligomerisation domains (NODs).
These NOD receptors not only recognise intracellular pathogen-associated molecular patterns (PAMPs), but also self-generated signals known as damage-associated molecular patterns (DAMPs). Examples are extracellular ATP, uric acid, and heat shock proteins that accumulate with stress and trigger inflammasome activity. New evidence has placed inflammasomes at the centre stage of complex diseases (metabolic syndrome and carcinogenesis) and physiological processes (regulation of intestinal microbial ecology) and energy management.22-28
Increased toxic metabolites and transmembrane ion leakage suppresses the core ability of the mitochondria to produce ATP and alter nutrient uptake, resulting in overall reductions in energy and persistent fatigue.









