


July 2004
by Dr. Nigel Plummer
Introduction
It is commonly assumed that the decline in gastric acid production observed
through later adult life (approximately 30% of the population over 65 years
are hypochlorhydric) is a "normal" and very common consequence
of the ageing process. Recent evidence, however, shows that these assumptions
are incorrect and that the frequently observed reduction or loss of gastric
acid production is generally the result of often-undiagnosed asymptomatic
atrophic gastritis. The atrophic gastritis often has underlying infections
as its root cause.
This mini review describes the prevalence and causes of atrophic gastritis
and its linkage with achlorhydria, and then outlines protocols for how the
disease can be resolved and prevented from recurring. As the most common observed
symptom of atrophic gastritis is probably hypochlorhydria, it is important
to review the basic physiology of gastric acid production.
Physiology of Gastric Acid Production
The physiological role of gastric (hydrochloric) acid may be summarized as:
1) Initiation of protein breakdown through activation of pepsinogen
2) Augmentation of nutrient absorption, notably dietary calcium and iron
3) Provides a barrier effect of entry of microorganisms to the GI tract.
Approximately 2 liters of gastric juice are produced per day, stimulated by
a combination of vagus nerve excitation and production of gastrin, acting to
stimulate the release of histamine. This in turn causes parietal or oxyntic
cells to produce hydrochloric acid. Parietal cells are located in the fundus
and body sections of the stomach (Figure 1).
Figure 1. The Main Anatomical Features of the Stomach (adapted from Smith & Moreton, 2001)
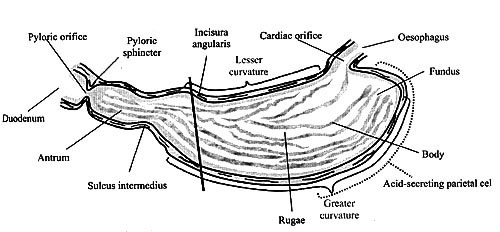
The diagonal line (\) shows the approximate division of the stomach into the two secretory regions: the oxyntic secretory area consisting of the fundus and the body, and the pyloric secretory area consisting of the pyloric antrum.
Gastric Acid Release in Response to Food
HCl is secreted at approximately 10% of maximal rate into the resting stomach
and, with nothing to neutralize the acid, maintains the pH in the range of
1.8-2.8. In the empty stomach the hydrogen ion concentration provides a feedback
loop that stops production of acid once a pH of 1.8-2.0 is reached, so preventing
over-acidification.
The sensory stimuli, which are activated just prior to eating, provoke strong
release of gastric juice depending on the quantity and type of food eaten.
However, this is balanced upon ingestion, because the food itself has a buffering
or neutralizing effect on the pH value. This typically results in the pH of
the full stomach being between 3.5-4.5. Following a meal the pH decreases once
more back to empty stomach level of 1.8-2.8.
Figure 2. Hourly intragastric acidity in nine subjects eating six normal meals during a 24-hour period (Redrawn from Pounder & Frazer, 1993)
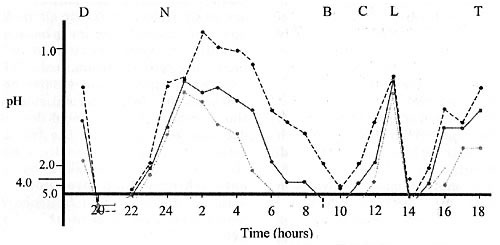
Key: D=Dinner; N=Nightcap; B=Breakfast; C=Coffee; L=Lunch; T=Tea
Effects of Age on Gastric Acid Secretion
It has long been the belief that the production of gastric acid decreases steadily
with age — especially after the age of 45. Indeed, virtually all studies
up to 1970 showed a decline in basal and stimulated acid production with
increasing age (Blackman et al., 1970). Contrary to this, recent studies
have indicated that in the normal stomach, the secretion of gastric acid
does not decrease with age, and the trend is actually to increase, especially
in men. However, this is masked by the astonishingly high prevalence of atrophic
gastritis, and Helicobacter pylori infections, both of which have been found
to be associated with secondary hypochlorhydria in older individuals (Goldschmiedt
et al., 1991; Greenwald & Brandt, 2003).
Atrophic Gastritis
Atrophic gastritis is typically a non-erosive gastritis where inflammation
is associated with a loss of function of the secretary cells of the fundus
and antrum sections of the stomach. The level of inflammation tends to be
variable, with infiltration of macrophages and lymphocytes into the mucosa
being either very low and patchy or uniformly high.
The prevalence of atrophic gastritis is remarkably high. Siurala et al. (1960)
reported a 28% prevalence rate in 142 randomly selected subjects between 16
and 65 years of age. Similarly, incidence rates of 20% and 28% have been found
on similar studies in healthy adults (Villako et al., 1997; Kreuning et al.,
1978). All of these studies noted a marked increase with age, which was confirmed
by Krasinsky et al. (1986) who found an incidence of 31.5% in a free living,
largely asymptomatic population between 60-99 years of age.
A summary of the data strongly suggests an incidence of atrophic gastritis
of greater than 15% in adults above 25 years and greater than 30% in people
older than 60.
Symptoms and Causes of Atrophic Gastritis
The progression of atrophic gastritis can occur over a period of years and
can be co-presented with erosive gastritis especially if there is regular
use of non-steroidal, anti-inflammatory drugs (NSAIDs). Often the
disease is largely asymptomatic but nonetheless, sufferers will often complain
of intermittent dyspepsia, abdominal pain, distention or bloating, and nausea
or vomiting. In addition, there are related sequelae resulting from the atrophy
of the functional components of the stomach. Thus, loss of parietal cells
causes reduction in acid production and intrinsic factor, the latter making
vitamin B12 deficiency common.
The most common cause of atrophic gastritis and hence of lowered stomach
acidity is chronic infection by Helicobacter pylori. This organism is the
most common
chronic bacterial pathogen in humans and is also a common focus of chronic
inflammation, which is increasingly implicated in progressive, often intractable,
diseases such as atherosclerosis and age related mental disorders such as Alzheimer's
disease, depression and bipolar disorder. In addition, the symptoms synonymous
with the chronic elevation of the pro-inflammatory cytokines TNF, IL-1 and
IL-6 resulting from prolonged H. pylori infection are precisely the same symptom
manifestations as seen with chronic fatigue and chronic pain syndromes such
as chronic fatigue syndrome and fibromyalgia. Hence, chronic infections such
as H. pylori may cause, or be a strong contributor, to these diseases.
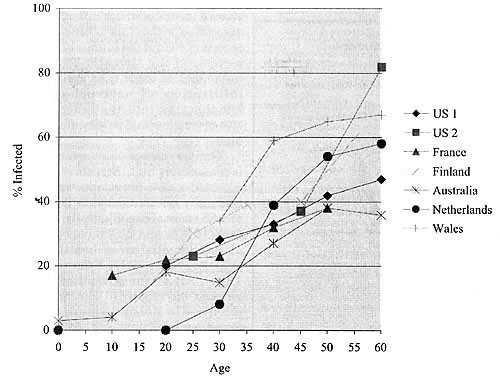
In general, the prevalence of H. pylori infection increases 1% for every year
of life, and so 50% of 50 year olds are typically infected. This picture is
similar for most industrialized countries, with populations of developing countries
showing even greater frequency (Figure 3) resulting from high infection rates
in children and young adults.
Interestingly, as well as H. pylori being responsible for reduction in gastric
acidity via atrophic gastritis, it is also now recognized that the initial
infection with the bacterium probably takes place only when the acidity level
in the stomach is decreased, albeit even on a temporary basis. Thus in two
human inoculation experiments, infection could not be established unless the
pH of the stomach was raised by use of histamine antagonists (Marshall et al.,
1985; Morris & Nicholson, 1987). Indeed, it is now known that H. pylori,
like most microorganisms, is sensitive to gastric acid, but avoids the strongly
acidic environment of the lumen by infecting when acid output is temporarily
lowered (a common occurrence) and then migrating below the mucous layer in
contact with the epithelium. In this way, it protects itself from acid output
once it becomes normalized.
The increasing incidence of H. pylori infection with age indicates that once
infection is established, it is persistent, perhaps lifelong in many cases,
and that it clearly survives normal antibiotic therapy. The clinical outcomes
of chronic infection with H. pylori are diverse but it is now estimated that
in individuals with unresolved chronic infection:
- 80% will demonstrate atrophic gastritis
- 20% will develop duodenal ulcers
- 10% will develop gastric ulcers
- 1% will develop gastric/duodenal cancer
Moreover, of all cases of duodenal and gastric ulcers, an estimated 90% and 65% respectively are caused by H. pylori (Parsonnet, 1998). Interestingly, the majority of the other gastric ulcers are caused by long-term use of NSAIDs. The reason for the development of disease in some individuals and not others is unclear but smoking and excessive alcohol consumption are known risk factors.
Figure 3. Prevalence of Helicobacter pylori infection by age in industrialized countries (from Taylor & Blaser, 1991)
Once diagnosed, usually by biopsy and urease-positive testing, or by ELISA
identification of IgG specific antigens, Helicobacter infections are treated
by antibiotics. One of the most common combinations is metronidazole, tetracycline
and bismuth for 2 weeks. This provides eradication in 70-90% of cases but
the symptoms of gastric disturbance can continue for a minimum of 12 months
thereafter.
Similarly, in terms of atrophied gastric mucosa, there is no confirming evidence
that eradication of Helicobacter pylori results in resumption of normal gastric
acid secretion and so there is a strong likelihood of permanent reduction in
acid production following many cases of chronic infection.
Hypochlorhydria and Achlorhydria
A significant consequence of atrophic gastritis is hypochlorhydria and achlorhyria,
which in turn may have the following effects on physiology (Howden & Hunt,
1987; Modlin et al., 1994):
1) Increased microbial enteric infections and small intestinal bacterial overgrowth
2) Increase in intestinal permeability resulting from malabsorption and/or
bacterial overgrowth or alteration of gastric mucosa architecture as a result
of low acidity levels
3) Nutrient malabsorption.
Intestinal Infection
It is well recognized that reduction in stomach acidity increases risk of infection
by Salmonella, Shigella, E. coli, Vibrio cholerae and the protozoan Giardia
(Howden & Hunt, 1987). In developed nations where hygiene standards and
microbial water quality is high, this may be a relatively insignificant problem.
However, in developing nations, this is often not the case and increasing
levels of achlorhydria is seen as being a major contributor to the levels
of enteric infection and diarrhea (Howden & Hunt, 1987).
Similarly, it is known from animal experiments that low acid production increases
susceptibility to nematode and helminthes infections. This is unlikely to be
different for humans and may be one of the contributing factors to the enormous
prevalence of parasitic infections in both developed and developing countries.
Small Intestine Bacterial Overgrowth
Overgrowth of bacteria in the stomach, duodenum and jejunum is commonly associated
with hypochlorhydria, especially if chronically associated with atrophic
gastritis. The overgrowth organisms are typically Staphylococci, Streptococci,
Enterococci, and Candida and they probably originate from oral source rather
than "growback" from the ileum and large intestine. The normal
fragile flora of Lactobacilli typically found in the healthy small intestine
appears to be overwhelmed (Husebye et al., 1992). Because these organisms
are considered commensals, acute adverse effects from their overgrowth are
rare. However, the overgrowth of potentially antibiotic resistant Staphylococci
and Enterococci is never desirable, and may have marked adverse effects in
the immunocompromised or those intending to have intestinal surgery.
In addition to this, there is a growing body of opinion and data that suggests
that dysbiosis in the intestine can be responsible for, or be a significant
contributor to, irritable bowel syndrome, chronic fatigue syndrome, arthralgias
and depression (Madden & Hunter, 2002).
Long-term chronic sequelae to bacterial overgrowth are likely to become more
recognized as studies accumulate, and indeed our own research group found in
open human invasive trials that mucosal colonization by Candida albicans and
other yeasts is very common whenever intestinal overgrowth with these fungi
occurs (Madden et al. — in press). The importance of the mucosal colonization
by the yeast was that it triggered a change in morphology from the single cell
to hyphal form of the yeast. This in turn elicits an inflammatory response
in the mucosa, which if not resolved could give rise to a chronic inflammatory
condition.
![]()
Nutrient Malabsorption
Macronutrients
Possibly as a result of the overall over-consumption of food by the population,
the lack of gastric acidity seems to have little effect on absorption of fat
and carbohydrates (Saltzman et al., 1994).
However, the reduction in acid production has a direct effect on reducing the
production of pepsin, which is converted from the precursor pepsinogen by hydrochloric
acid (Modlin et al., 1996). Although gastric pepsin is responsible for between
20-25% of protein digestion, it is likely that in most but not all circumstances,
the production of proteases by the pancreas in younger adults compensates for
the gastric pepsin deficiency (Smith & Morton, 2001). However, the age-related
decrease in production of pancreatic enzyme output together with the lack of
gastric pepsin is certain to be a major contributory factor in the increasing
levels of protein and amino acid inadequacy seen in people as they age past
50 years (Greenwald & Brandt, 2003).
Achlorhydria is known to have profound effects on the absorption of some micronutrients,
the most important of which are described below.
Vitamin B12 and Folic Acid
Perhaps the most obvious nutritional consequence of achlorhydria associated
with atrophic gastritis is the resultant malabsorption of vitamin B12. The
major mechanism of this effect is that the acid-producing parietal cells
also produce intrinsic factor and once atrophied, this capacity is lost (Glass,
1963). In addition, however, lack of acid may inhibit the liberation of B12
from other nutrient components in food and bacterial overgrowth microorganisms
may compete for the B12 that is available (Schade & Shilling, 1967; Simon & Gorbach,
1984). The metabolism of vitamin B12 and folic acid is related, in that vitamin
B12 is necessary for the incorporation of folic acid into human tissue. This
level is reflected in red blood cell level of folate, and not serum levels,
which simply indicates the recent dietary intake of folic acid. This relationship
between these two nutrients means that vitamin B12 deficiency is a very strong
predictor of simultaneous deficiency in folic acid.
While the extreme deficiency associated with pernicious anemia is relatively
rare, the common epidemiological finding of sub-clinical deficiency of folic
acid vitamin-B12 in 15-40% of the population, especially in the elderly, is
undoubtedly due to the under-recognized and under-diagnosed level of atrophic
gastritis (National Diet & Nutrition Survey, 1994/5).
Other Nutrients
Insufficient acid production leads to an increase in small intestinal pH (Krasinski
et al., 1986) and this has been shown to cause reduction in absorption of
calcium, inorganic iron, and vitamins A and E (Recker, 1985; Mackenzie & Russell,
1976; Hollander, 1980; Muralidhara & Hollander, 1977).
Alteration of Intestinal Permeability
It would be expected that atrophy of the gastric mucosa and consequent bacterial
overgrowth in the small intestine would have a combination effect on intestinal
permeability. This is indeed the case, with both transcellular and paracellular
permeability, as measured by mannitol and lactulose uptake respectively,
being substantially increased in atrophic gastritis sufferers compared to
control patients (Salzman et al., 1994).
In addition, the clearance test for the acute phase protein alpha-antitrypsin
was also numerically higher in the atrophic gastritis group indicating the
presence of systemic reaction to inflammation. By implication, this means that
the mucosal barrier has been breached in these people with the probability
of significant translocation of microorganisms and dietary antigens into the
systemic environment.
Atrophic Gastritis — Prevention
and Treatment
Fortunately the prognosis of both prevention and treatment for atrophic gastritis
is very good, which is comforting given the frequency of the disease. All
treatment programs both for clinical and sub-clinical cases require the same
basic approach:
1) Eradicate the underlying cause of the atrophic gastritis
2) Manage the symptoms resulting from the gastritis while the long term healing
process takes place.
Eradicating the Cause of
Atrophic Gastritis — Helicobacter
pylori
The two most common causes of atrophic gastritis are infection with
Helicobacter pylori — approximately 65%, and chronic use of NSAID's — approximately
30% (Parsonnet, 1998). Helicobacter pylori infection is not successfully
resolved with normal antibiotic therapy, but combination use of antimicrobials
and other drugs are successful in eradication. Thus the most widely used
therapies currently are:
1) 2 weeks metronidazole, tetracycline and bismuth (>70% eradication)
2) 2 weeks of omeprazole with clarithromycin or amoxicillin (>50% eradication)
3) 1 week of omeprazole with clarithromycin and either metronidazole or amoxicillin
(>90% eradication).
The Effect of Plant Antimicrobials
A different approach to the use of antibiotics has been investigated by researchers
at the University of Wolverhampton in Great Britain, who have assessed the
potential for use of plant antimicrobials in treatment of Helicobacter infections.
They found that three different strains of H. pylori were highly sensitive
to some mixtures of essential oils, particularly oregano. The most effective
was a mixture of oregano, clove, wormwood and ginger oils. A summary of their
results is shown below (Table 1). It can be seen that the Minimum Inhibitory
Concentration (MIC) and Minimum Bactericidal Concentration (MBC) lies between
17-47mcg/ml and 35-110mcg/ml respectively for all strains tested.
N.B. MIC is the minimum concentration of antimicrobial agent necessary to inhibit
the growth of 1.0 million/ml of the target microorganism. MBC is the minimum
concentration of antimicrobial agent required to kill the target organism,
to a viable count of less than 100 cfu/ml.
Table 1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of a mixture of oregano, clove, wormwood and ginger oils against three strains of Helicobacter pylori — compared to E. coli
| Strain | MIC of Pyloricin | MBC of Pyloricin |
| Strain 11657 | 47mg/ml | 110mg/ml |
| Strain Roberts | 43mg/ml | 97mg/ml |
| Strain 106552B | 17mg/ml | 35mg/ml |
| E. coli | 430mg/ml | 960mg/ml |
These results confirmed that H. pylori is particularly
sensitive to essential oils, especially when compared to E. coli, which
is far less
susceptible to this particular mixture and requires a different approach
for effective in-vivo control.
Following the release of this data, Pharmax developed a product, Pyloricin,
containing the same mixture of oils. In addition, in this product, the oil
mixture is emulsified so that once in the stomach it produces a milky emulsion,
enabling the antimicrobials to effectively disperse and penetrate the mucous
layer and attack the surface and crypt-bound H. pylori cells.
Feedback from practitioners throughout the USA and UK indicates that eradication
rates with Pyloricin is >70% and this is now being confirmed in independent
human clinical trials using the radioactive labeled urea/urease breath test,
which is rapidly becoming the gold standard for diagnosis.
Management of Residual Gastritis
In most cases, the residual cellular atrophy and gastritis affecting the stomach
mucosa, repairs itself following eradication of H. pylori, but this process
can take many months or even years. Thus, it is important to manage the deficiencies
in intestinal function during this period of repair, and this can be achieved
best by targeted supplementation.
Supplementing HCl and Pepsin
Probably the most common consequence of atrophic gastritis, even if it is slowly
resolving, is the state of hypochlorhydria. The repercussions of the hypochlorhydric
state are outlined above, and if you add the likelihood of re-infection by
H. pylori onto this list, then the need for re-acidification of the stomach
by supplemental means is very persuasive.
How Much HCl and Pepsin Should be Provided?
HCl is usually supplied by providing betaine hydrochloride, which dissociates
in water to release betaine and free HCl. A 0.5% or 500mg/100mls aqueous
solution of betaine hydrochloride has a pH of 2.0. This means that to acidify
400mls of gastric juice to a level of pH 2.0 prior to eating, 2,000mg of
betaine hydrochloride is required.
Pepsin is also supplied alongside the HCl to ensure that sufficient proteolytic
activity is present in the stomach. The level of pepsin contained in a combined
product should be matched so as to provide sufficient activity to break down
approximately 3 grams of protein per 600mg of added betaine hydrochloride.
In all cases, it is prudent to begin supplementation to an individual in steadily
increasing levels. So, if a capsule of a product contains say 600mg of betaine
HCl, then an individual should start with one per meal and then build up to
3 capsules per meal, which will be the level required in the typical chronically
hypochlorhydric patient.
Combined HCl and pepsin supplements should be given prior to a meal to provide
both acid and pepsin augmentation. However, it is important to maintain basal
acidity in the empty stomach to provide the bacteriostatic barrier. In this
case, it is better to take the supplement without pepsin, as excessive pepsin
in the empty stomach can cause irritation of the mucosa.
Nutritional Consequences
The lack of intrinsic factor production resulting from atrophic gastritis will
substantially reduce the level of vitamin B12 absorption. The route of absorption
via intrinsic factor begins with the release of vitamin B12 from food protein
complexes by pepsin. The B12 then binds to glycoproteins called R-proteins,
which transport the B12 into the duodenum. Here, the complex is degraded
by pancreatic protease, releasing free B12 once more, which this time binds
to intrinsic factor produced in the stomach. In this conjugated form, the
B12 travels to the terminal ileum where it is absorbed by active transport.
However, approximately 1-2% of vitamin B12 is passively absorbed in the intestine
without the need for intrinsic factor (Smith & Morton, 2001) and as such,
if doses of 1mg or more per day are delivered, then between 10-20mcg will be
absorbed.
Hence a good quality multivitamin, providing 1mg or more of B12 per dose, should
be sourced and will be sufficient in most cases. If severe B12 deficiency is
suspected, then initial intravenous dosing to build up tissue levels could
be considered.
Resolution of Bacterial Overgrowth and Mucosal Conditioning
The bacterial overgrowth in the stomach and duodenum will begin to resolve
naturally with the re-establishment of acid production and/or supplementation
with betaine HCl. However, it is appropriate to accelerate this process by
use of plant antimicrobials to eliminate existing overgrowth and replace
with the normal lactic acid bacteria-dominated flora in the small intestine.
To achieve this, the antimicrobials allicin from garlic, cinnamaldehydes
from cinnamon, and also berberine from goldenseal have been found to profoundly
inhibit overgrowth organisms such as Staphylococci, Coliforms, and yeasts
without affecting Lactobacilli and Bifidobacteria (Rees et al., 1993). The
inhibitory effects of allicin against Salmonella, Staphylococcus aureus and
E. coli are visually depicted in Figure 4.
Figure 4. The anti-bacterial activity of allicin
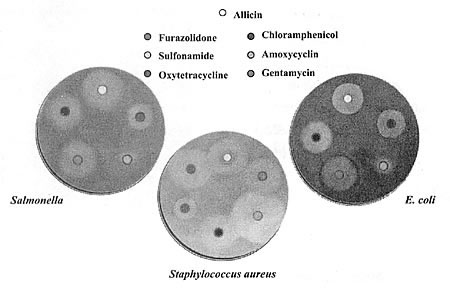
Table 1. MIC of allicin against various microorganisms
| Microrganism | MIC at 24 hours(mg/ml) |
| Staphylococcus aureus | 26 |
| Escherica coli | 44 |
| Candida albicans | 36 |
| Saccharomyces cerevisiae | 49 |
| Bacillus cereus | 47 |
| Listeria monocytogenes | 60 |
| Salmonella enteritidis | 62 |
| Pseudomonas aeruginosa | 237 |
| Lactobacillus sp | 390-520 |
| Enterococcus sp. | 650-1820 |
The antimicrobial nature of berberine against microorganisms such as E. coli and Candida albicans is shown in Table 2, as well as the lack of similar effect against the lactic acid bacteria, Lactobacillus plantarum and Lactobacillus acidophilus.
Table 2. MIC of berberine against various microrganisms
| Organism | MIC @ 24 hours (mg/ml) |
| E. coli | 120 |
| Candida albicans | 165 |
| Lactobacillus plantarum | 1,100 |
| Lactobacillus acidophilus | 2,400 |
Products containing these antimicrobials should
be taken with a high potency probiotic for a minimum of 60 days alongside
the administration
of HCl.
Finally, glutamine and arginine should be taken to help recondition the mucosal
architecture and underlying immune system.
Conclusion and Summary
- Atrophic gastritis affects 20-30% of the adult population
- Incidence increases with age
- Atrophic gastritis is highly associated with Helicobacter pylori infection
- Hypochlorhydria is probably the most common symptom of atrophic gastritis
- Gastric acid secretion does not decrease with increasing age in healthy (non atrophic gastritis) individuals
- Hypochlorhydria can result in increased risk of enteric infections, nutrient malabsorption, bacterial overgrowth and dysbiosis, and increased permeability of the intestine
- Atrophic gastritis is a strong risk factor for gastric and duodenal ulcers and gastric cancer
- Effects of atrophied gastric mucosa remain for months or years after eradication of H. pylori
- Resolution
of atrophic gastritis and symptoms depends upon:
a. elimination of H. pylori
b. treatment of residual atrophic gastritis.
References
Blackman AH, Lambert DL, Thayer WR & Martin HF. 1970 Am. J. Dig.
Dis. 15 (9): 783.
Glass GBJ. 1963 Physiol. Rev. 43: 529.
Goldschmiedt M, Barnett CC, Schwarz BE, Karnes WE, Redfern JS & Feldman
M. 1991 Gastroenterol. 101 (4): 977.
Greenwald DA & Brandt LJ. 2003 In
Geriatric Medicine and Gerontology. ISBN
0443070873.
Hollander D. 1980 Am. J. Physiol. 239:
210
Howden CW & Hunt RM. 1987 Gut 28:
96.
Husebye E, Skar V, Hoverstad T & Melby K. 1992 Gut 33:
1331.
Krasinski SD, Russel RM, Samloff IM, Jacob RA, Dallal GE, McGandy RB & Hartz
SC. 1986 J. Am. Geriatr. Soc. 34: 800.
Kreuning J, Bosman F, Kuiper G, Van der Wal A & Linderman J. 1978
J. Clin. Pathol. 31: 69.
Mackenzie JF & Russel RI. 1976 Clin. Sci. Mol. Med. 51:
363.
Madden JAJ & Hunter JO. 2002 Brit. J. Nutr. 88
(1): S67.
Marshall BJ, McGechie DB, Rogers PA & Glancy RJ. 1985 Med.
J. Aust. 142: 439.
Modlin IM., Goldenring JR, Lawton GP & Hunt R. 1994 Am. J.
Gastroenterol. 89 (3): 308.
Morris A & Nicholson G. 1987 Am. J. Gastroenterology 82:
192.
Muralidhara KS & Hollander D. 1977 J. Lab. Clin. Med. 90:
85
Parsonnet J. 1998 In Infectious Diseases. ISBN
072166119X
Pounder RE & Fraser AG. 1993 Baillere's Clinical Gastroenterology 7
(1): 55.
Recker RR. 1985 N. Engl. J. Med. 313
(2).
Rees LP, Minney S, Plummer NT & Slater J.H. 1993 World J.
Microbiol. Biotechnol. 9: 303
Saltzman JR, Kowdley KV, Pedrosa MC, Sepe T, Golner B, Perrone G & Russel
RM. 1994 Gastroenterol. 106: 615.
Schade X & Schilling X. 1967 Am. J. Clin. Nutr. 20:
636.
Simon GL & Gorbach S.L. 1984 Gastroenterology 86
(1).
Siurala M, Eramaa E & Nybers W. 1960 Acta Med. Scand. 166:
213.
Smith ME & Morton DG. 2001 In The
Digestive System ISBN
0443062455.
Taylor DN & Blaser MJ. 1991 Epidemiol. Rev. 13:
50
Villako K, Tamm A, Savisaar E & Ruttas M. 1997 Scand. J. Gastro. 11:
817.
Search
our pre-2001 archives
for further information. Older issues of the printed magazine are also
indexed for your convenience.
1983-2001
indices ;
1999-Jan. 2003 indices
Once you find the magazines you'd like to order, please use our
convenient form, e-mail subscriptions@townsendletter.com,
or call 360.385.6021 (PST).
All rights reserved.
Web site by Sandy Hershelman Designs

