|
Does this clinical scenario sound familiar? The patient has food sensitivity and suspects that she may be gluten intolerant. She is advised to avoid wheat products and other grains, and sent home to get healthy. Yet I hear of case after case in which patients with severe gluten sensitivity have been put on a gluten-free diet for two to three months with little or no improvement. Why? There are a number of issues involved in managing gluten sensitivity and celiac disease (CD).
If we measure antibodies against only one component of wheat (alpha-gliadin), we will miss 50% of individuals with gluten sensitivity.
- Individuals with gluten sensitivity are often highly reactive to other foods: for example, in 50% of cases, there is reactivity between wheat and dairy proteins.
- Among those with celiac disease, we know that 50% of patients do not react to the protein that causes reactivity in everyone else.
- Many celiac patients do not produce antibodies against transglutaminase 2, but rather against transglutaminase 3 or 6.
Today gluten sensitivity is projected to affect three people in every 100.1 Celiac disease is estimated to affect almost one in every 100,2 and of those with digestive disorders, it affects approximately one in 50. Projections of CD's prevalence range from 1.8 million to 3 million individuals in the US alone, with as many 1.4 million people who have undiagnosed celiac disease. According to researchers, for every patient diagnosed with this disorder, there are another eight individuals undiagnosed with CD who have no apparent gastrointestinal symptoms.3-6 These patients are often diagnosed more than four years after they become fully symptomatic. The percentage of individuals over 20 years of age with CD who go on to develop autoimmune conditions is estimated at one in three.7 Some clinicians believe these risks are even greater.
In response to a virtual epidemic of autoimmune disorders, our laboratory has committed substantial resources to the development of highly reliable evaluations for gluten and food sensitivities and celiac disease. Our goal is to provide clinicians with state-of-the-science tools to reduce patient suffering through reliable testing that supports evidence-based treatment.
Increasing Test Accuracy
We have applied decades of research and laboratory experience to develop protocols that assure accurate testing and reproducible results.
Assaying in duplicate. We perform each test in duplicate samples; the probability of having the same error occur in replicate samples simultaneously is close to zero. In the protocol used at Cyrex, when samples correlate within plus or minus 20%, then the results are reported. If the samples do not correlate, the assay will be repeated with replicate samples until there is correlation within the same run, and only then will those results be reported.
Utilizing pure antigens. To achieve good reproducibility, laboratories must use absolutely pure antigens in the ELISA evaluation. Consider the example of measuring antibodies against broccoli: many labs either purchase broccoli powder or prepare a sample medium themselves in the lab by emulsifying broccoli in a food processor. The problem is that there are many inhibitors in either type of testing medium, including numerous enzymes, and large and small molecules. None of these substances are relevant to the protein in broccoli. As a result, tests using impure antigens frequently produce false positives and false negatives. To achieve reproducible results, the protein in broccoli must be extracted and purified in order to measure antibody activity against that particular protein.
Testing for both IgG and IgA. In the past, testing for delayed food sensitivity was offered only in IgG. From a scientific perspective, the combined IgA and IgG evaluation is potentially a more accurate blood test for food reactivity, with greater reproducibility. Double-blind research studies have shown that labs do not always demonstrate good reproducibility in IgG food testing. At Cyrex, we decided from both a scientific and a management perspective to combine the two tests and provide both for one value.
IgA as the first line of defense. Textbooks on mucosal immunology indicate that when the body is exposed to an antigen via the digestive tract, due to the nature of lymphocyte response, the body will produce antibodies of IgA isotype first, before IgG or IgM. Some of the antibodies from the salivary glands are secreted into the blood, so IgA can be detected first in saliva and then in blood. If the patient consumes more and more of the same antigen, an inflammatory reaction may occur in the gut, causing the tight junctions to open. Antigens flood into the submucosa; from there they migrate into the regional lymph nodes and then into the circulation. At this point the body will begin producing IgM or IgG antibodies against those food antigens.
Relevance of IgA in diagnostic evaluation. Consider recent examples from diagnostic medicine on the importance of IgA. In cases of celiac disease, IgA antibodies against both gliadin and transglutaminase are diagnostic markers, both in saliva and in blood. In evaluations for Crohn's disease, anti-saccharomyces IgA antibodies serve as a diagnostic marker with a high degree of correlation. In immunoparasitology, IgA antibodies are diagnostic, rather than IgG. Generally speaking, when exposure to an antigen occurs in the digestive tract, the immune response will be in the form of IgA first and IgG later. These examples from the research demonstrate the diagnostic relevance of IgA when measuring delayed immune responses to food antigens.
Straightforward Gluten Issues
As a nutritionist, obviously I begin by taking a history to better define the patient's situation, what he has been eating, what he has tried, and what he has not tried, including past testing. When the cost of testing is an issue for clients, I opt for a basic saliva test (Array 1). If they have had a previous gluten test (IgG) that was negative, or if they are still eating gluten-containing foods and they continue to have a problem, that is strong justification for ordering a comprehensive gluten panel (Array 3). My goal is to completely eliminate the possibility that gluten is an issue. If they test positive and they cut out gluten and still do not respond, then I order the panel for cross-reactivity (Array 4).
Jerry Stine, NC, San Francisco Bay Area
The breakdown of mucosal immune tolerance results in GALT (gut-associated lymphoid tissue) reactivity to luminal antigens. These reactions can trigger the production of IgA and IgM antibodies against gliadin, tissue transglutaminase, and endogenous bacteria, as well as an increase in pro-inflammatory cytokines, evident in oral fluids of the body. Saliva is a source of body fluid conducive to the detection of an immune response (90% IgA and 10% IgM) to food, bacterial, and other antigens present in the oral cavity and gastrointestinal tract. Indeed, salivary antibody induction has been widely used as a model system to study secretory responses to ingested material, particularly because saliva is easy to collect and analyze. Elevated antibodies targeting gliadin and transglutaminase can be measured in saliva, providing an efficacious basic screening tool with a number of advantages:
- Cost-effective early detection
- Highly sensitive assessment before the onset of gut damage
- Ease of salvia sampling, particularly for children and the elderly
- Scientific justification and motivation for a gluten-free diet
- Affordable periodic monitoring for patients with a family history of celiac disease
- Enhanced testing for IgA and IgM
- Improved methodology to maintain sample quality
- More accurate test results
Saliva tests are designed to accurately inform health care providers of clinical disorders by detecting the breakdown of immune tolerance and facilitating the diagnosis of patients with gluten intolerance or CD. Ongoing longitudinal research by Bonamico followed 5,000 children with CD using salivary assessments for gluten reactivity. Results showed stronger correlation in saliva than serum before villous atrophy occurred and higher correlation in serum assessments once total villous atrophy was present.8
Testing for Leaky Gut
Reactivity to wheat/gluten fractions is a known cause of gut inflammation and intestinal permeability, and is often associated with autoimmune diseases as a trigger antigen or an exasperator of the clinical condition. Whenever gluten sensitivity is identified, it is crucial to establish a functional baseline for the degree of intestinal permeability. This increased permeability is also a gateway to autoimmunity and must be addressed in all patients presenting with inflammatory autoimmune conditions.
By testing for antibodies to wheat/gluten fractions, bacterial toxins, and intestinal barrier proteins, the clinician can assess quantitatively the level of damage to this barrier and the effectiveness of the treatment protocol (Array 2). Use of cost-effective permeability testing makes it possible to provide accurate initial screening and periodic ongoing monitoring to determine the success of therapy.
Array 2. Intestinal Antigenic Permeability Screen
Actomyosin IgA, IgG
Occludin/Zonulin IgG, IgA, IgM
Lipopolysaccharides (LPS) IgG, IgA, IgM
Comprehensive Testing for Wheat/Gluten Reactivity
Periodically physicians inquire about the utility of the additional information provided by in-depth testing for reactivity to wheat and gluten fractions. Based on the research literature and our own evaluations, we have found that this testing serves a number of highly important functions.
Accurately diagnosing gluten sensitivity. We will miss 50% of individuals with gluten sensitivity if we measure antibodies against only one component of wheat (alpha-gliadin). When gluten reactivity goes undiagnosed, the patient continues to consume substances that are toxic to his or her biochemistry, which will eventually result in autoimmunity.
Consequences of untreated gluten reactivity. Gliadin has been shown to cross-react with multiple organs and tissues, including cerebral function (reflected in impact on gangliosides and synapsins), and effects on thyroid and on reproductive organs. This reactivity has been shown to contribute to a range of health conditions, including neurological dysfunction, thyroiditis, and infertility.
Diagnosing celiac disease. There is a significant possibility of missing celiac disease in laboratory testing. In research conducted in 2005, I found that although 50% of patients react to alpha-gliadin, the other 50% react to a completely different set of antigens or peptides such as omega-gliadin, gamma-gliadin, glutenin, gluteomorphin, prodynorphin, or wheat germ agglutinin.
Table 1: Antigenic Profiles of Gluten and Celiac Disorders
Exposure to environmental factors
(rota virus, bacterial endotoxins, medications) |
| Breakdown in mucosal immunological tolerance against gliadin |
| Non-HLA DQ2/DQ8-inherited |
Genotype HLA DQ2/DQ8+ |
| Gluten Sensitivity |
Celiac Disease |
| Inflammation in the absence of transglutaminase (tTG) activation and no evidence of villous atrophy |
Inflammation and activation of tTG and damage to the villi; evidence of villous atrophy |
| No deamidiation of gliadin peptides |
Deamidiation of gliadin peptides and binding to tTG |
| Production of more IgA and/or IgG against native gliadin and other gluten-related autoantibodies in the blood |
Production of IgA and/or IgG against deamidiation gliadin peptide, tTG, and gliadin-tTG complex |
| Cross-reaction with different tissue antigens, creating risk of complications with various autoimmune conditions |
Various autoimmunities and cancer |
Differentiating celiac disease from gluten sensitivity. To treat our patients successfully, we need to be able to differentiate between various gluten-related disorders. "The Oslo definitions for celiac disease and related terms"9appeared in Gut on February 16, 2012, and has been available online. This review, which evaluates the major issues in gluten sensitivity, introduced the term non-celiac gluten sensitivity. In these conditions, laboratory results are negative for IgA against transglutaminase, and positive for IgG or IgA against gliadin. In non-celiac gluten sensitivity, symptoms are triggered by gluten ingestion, with the absence of enteropathy and no evidence of tissue transglutaminase antibodies. These patients are usually HLA DQ2/DQ8 negative. Current laboratory diagnostic workup for celiac disease requires positive IgA antibodies against both transglutaminase 2 and gliadin, along with genotype HLA DQ2/DQ8 (see Table 1).
Celiac disease processes. The first step in the development of CD is the initial breakdown in immunological tolerance against various wheat proteins. In individuals with HLA DQ2/DQ8 genotype, environmental triggers activate tissue transglutaminase 2. Deamidation of gliadin epitopes by transglutaminase enables them to be presented with high affinity by antigen-presenting cells to helper T cells and then B cells, resulting in antibody production against gliadin, transglutaminase 2, and gliadin-transglutaminase complex. This process initiates a cascade of events resulting in mucosal inflammation, small intestinal villous atrophy, increased intestinal permeability to large molecules, and malabsorption of nutrients. Gliadin by itself is not water soluble; however, in the presence of transglutaminase, the amino acid glutamine converts to glutamic acid and therefore becomes water soluble. In many individuals this water solubility makes the gluten more digestible. However, in others, these water-soluble molecules have the tendency to complex with transglutaminase, which results in antibody production against this neoepitope.
Differentiating subsets within CD. In my own research published in the journal ISRN Allergy,10 I found that many individuals react to different components of wheat proteins. Earlier studies of intestinal T cells from gluten-sensitive/celiac disease patients showed a wide range of individual responses to wheat fractions. Similarly, I found that measurements of IgG and IgA antibodies against an array of wheat peptides and antigens can enhance the sensitivity and specificity of serological assays for gluten sensitivity and celiac disease, and may also detect silent celiac disease or its overlap with inflammatory bowel disease (IBD). Approximately 50% of patients with IBD appear to have gluten sensitivity. If gluten immune reactivity is not addressed in these patients, their clinical condition is not likely to improve.
Most clinical labs measure antibodies against transglutaminase 2, but not against other transglutaminases. However, many patients do not make antibodies against transglutaminase 2, but rather against transglutaminase 3 (according to the literature on dermatitis herpetiformis) or 6 (which has an impact on brain function, as in gluten ataxia). At Cyrex, we believe that it is just as vital to measure antibodies against multiple forms of transglutaminase as it is to measure antibodies against multiple fractions of wheat. We see many reports with negative test results for transglutaminase 2 that are positive for transglutaminase 3 or 6, with positive IgA or IgG response to gliadin proteins or peptides.
Array 3. Wheat/Gluten Proteome Reactivity and Autoimmunity
IgG and IgA against the following:
Wheat; Wheat Germ Agglutinin
Native and Deamidated Alpha-Gliadin 33-mer, Alpha-Gliadin 17-mer, Gamma-Gliadin 15-mer, Omega-Gliadin 17-mer, Glutenin 21-mer
Gluteomorphin and Prodynorphin
Gliadin-Transglutaminase Complex Transglutaminase 2, 3, 6
Early detection of complex conditions such as autism. It has long been my message that early detection can prevent the onset of many environmentally induced autoimmune conditions, including autism. Genetics have been implicated in a small percentage of autism spectrum disorders (ASD), while environmental triggers (or a combination of genetics and environment) have been well established in the biomechanisms that result in ASD. According to the literature, factors that can result in the development of autism in the offspring11 include the mother's exposure to pesticides or air pollution before conception, as well as obesity in the mother and advanced age of the father at time of conception. If these risk factors are present, taking a wait-and-see-approach to the diagnosis of gluten sensitivity is no longer appropriate. Early intervention is extremely important for the sake of the child.
In the ASD group and in patients with other gluten-triggered neurological conditions, we often see positive antibodies to opioid peptides such as gluteomorphin or prodynorphin. For this patient population, gluten has effects like an opiate drug that interferes with neurotransmitter messaging, so the implementation of a gluten-free diet early on has proven to be essential. Once patients are diagnosed with ASD or attention deficit disorder, the gluten-free diet has been shown to improve their condition without the use of medication.
Additional approaches to the management of these conditions include a GMO-free diet from birth and organically produced baby foods for these children. A report by the Institute for Responsible Technology (www.responsibletechnology.org) indicates that nearly all the infant formula on the market, including government distributions, contains GMO corn or soy, as well as milk from cows injected with bovine growth hormone.
Table 2. Cyrex Evaluations for Gluten and Celiac Reactivity
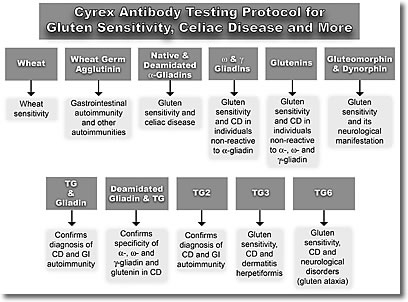
Causal factors in CD. The increasing number of patients with celiac disease is due in part to changes in wheat cultivars. This is not a GMO factor, but does reflect genetic engineering in agriculture. Changes have been introduced in the composition of modern wheat that were not present 50 or 100 years ago. For example, alpha-gliadin 9 content has been found to be much higher in current wheat varieties.12,13 This suggests that modern wheat breeding practices could be causing increased exposure to gliadin epitopes.
Due to these changes in the structure of wheat protein, our bodies no longer have the correct regulatory T cells to differentiate friend from foe, regulate immune activity, and minimize food antigen reactions. This breakdown of immunological tolerance also compromises the effective presentation of antigens, with an impact on antibody production. The peptides in earlier grain cultivars have been consumed by human populations for thousands of years. Now we have complexly configured grains that are new to the human population. Adults today developed oral tolerance to the wheat cultivars that were grown when they were children. The regulatory immune systems of adults may not recognize the new wheat proteins as friendly, and many individuals react adversely to these new peptides. The result is food sensitivity, which if not addressed, can cause autoimmunity. This is an example of why autoimmune conditions are increasing in aging populations.
The wheat-alcohol connection. Individuals who lack dipeptidyl peptidase IV (DPP IV) cannot digest gluten proteins and peptides. Consuming wheat-based foods and alcoholic beverages together greatly intensifies the impact of these antigens. In a recent study, researchers identified 150 different gluten peptides in beer. For a susceptible individual, consuming beer with a wheat product such as pizza overwhelms the digestive system with gluten peptides. In addition, the alcohol in the beer solubilizes a significant amount of the gliadin peptides in the pizza. As a result, the immune system may be overwhelmed by a flood of antigens (including gliadin) that affect tight junctions, trigger leaky gut syndrome, and cause the release of large molecules into the bloodstream and across the blood-brain barrier.
Testing for Cross-Reactive Foods
Patients who have not responded to the removal of gluten from their diet often benefit from testing for sensitivity to cross-reactive foods.Reports from clinicians indicate that a significant percentage of these patients improve dramatically with the reduction of cross-reactivity. For example, in approximately 50% of cases, there is reactivity between wheat and the proteins in dairy products (alpha-casein, beta-casein, and other milk proteins) and vice versa. Another example is the patient who substitutes grains such as millet for wheat. We received a recent report regarding a patient on a gluten-avoidance diet, who found that she was cross-reactive to millet at a level of 4+. When she removed millet from her diet, she improved significantly within a week. Accompanying reports like this, I frequently receive letters from doctors thanking me profusely for developing these tests.
To provide highly accurate testing at an affordable price, Cyrex now offers a cross-reactivity panel for 24 foods. As described earlier, a minimum of four assays are performed for each food, by twice sampling IgA and IgG antibodies for each food. These 24 foods are those most commonly consumed in a gluten-free diet, including foods that tend to be highly cross-reactive with gliadin. This panel provides a reliable basis for making nutritional recommendations that support exceptionally positive patient outcomes.
Treating unresponsive gluten-related conditions. When patients with known gluten sensitivity do not improve despite removing wheat from their diet, the tests for cross-reactivity provide a useful next step in the diagnostic process. Foods that cross-react in vivo to purified gliadin include gluten-containing grains (rye, barley, spelt, kamut®), dairy (cow's milk, casein, casomorphin, milk butyrophilin, whey protein, milk chocolate), oats, yeast (baker's and brewer's), millet, corn, and rice.14 A special note about chocolate: pure cocoa and milk-free dark chocolate have many beneficial characteristics. Only chocolate containing milk causes cross-reactivity with gliadin.
Copycat proteins. Dairy products are the most reactive of all these foods. Cross-reactivity is also predominant with yeast, corn, rice, millet, and oats. Some forms of these foods are completely safe, but others are cross-reactive. Some cultivars contain the same epitope, the same amino acid sequences that are found in wheat. Based on this understanding, oats should be on the list of cross-reactive grains.
Contamination in manufacturing. Food manufacturing processes also play a role in reactivity. Coffee provides a good example. Instant coffee is reactive at 20% to anti-gliadin antibodies, a significant finding that often reflects the presence of wheat fractions in the factory. Yet when we tested espresso from five different well-known coffee houses, we found no reactivity due to anti-gliadin antibodies. We also found no reactivity in tests on coffee beans purchased from Israel, Turkey, Greece, Colombia, and Brazil. Therefore, coffee beans do not appear to be cross-reacting with wheat, but some instant coffees are apparently cross-reactive due to contamination. These results indicate that drinking pure coffee, but not instant coffee, is safe for individuals with gluten sensitivity and celiac disease as long as these individuals do not have a classic allergy to coffee.
Array 4.
Gluten-Associated Cross-Reactive Foods and Foods Sensitivity
IgG and IgA combined against the following:
Rye, Barley, Spelt, Kamut®
Cow's Milk (Alpha- and Beta-Casein, Casomorphin, Milk Butyrophilin), Whey Protein, Chocolate (Milk)
Yeast, Egg
Oats, Buckwheat, Sorghum, Millet, Hemp, Amaranth, Quinoa, Tapioca, Teff
Sesame, Coffee, Soy, Corn, Rice, Potato
This next generation of comprehensive assays goes beyond conventional testing by providing a measurement of antibodies against multiple gliadins and transglutaminases, as well as cross-reactive foods. Multiple autoimmune reactions can develop in response to chronic inflammation caused by antibodies targeting dietary proteins and peptides and by cross-reactivity with various tissue antigens. These antibodies can now be documented in serum five to ten or even fifteen years before the onset of autoimmunity. By dealing with gluten reactivity, gut permeability, cross-reactive foods, and associated antibodies, we may be able to stop or even reverse the course of many autoimmune responses. In some cases, we now have the capacity to prevent autoimmune disorders years before they present as pathology.
 Aristo Vojdani, PhD, MSc, CLS Aristo Vojdani, PhD, MSc, CLS
Dr. Vojdani, Chief Scientific Advisor of Cyrex Laboratories, obtained his PhD in the fields of microbiology and clinical immunology with postdoctoral studies in tumor immunology at UCLA. His ongoing research, spanning a 40-year career, focuses on the role of environmental factors in complex diseases, including toxic chemicals and infection, as well as dietary proteins and peptides. Owner of 15 US patents for laboratory assessments, he has published more than 120 articles in the peer-reviewed literature, and serves in an advisory and editorial capacity for ten scientific journals. Dr. Vojdani is CEO and Technical Director of Immunosciences Lab Inc. in Los Angeles. His work with environmentally induced autoimmunity has culminated in next-generation laboratory assessments available at Cyrex Laboratories.
Cyrex Laboratories
Cyrex™ is a clinical immunology laboratory specializing in autoimmunity. The lab offers multi-tissue antibody testing for the early detection and monitoring of today's complex autoimmune conditions. Cyrex develops innovative arrays through continuous collaboration with leading experts in medical research and clinical practice. For additional information, see www.CyrexLabs.com.
5040 N. 15th Avenue, Suite 107
Phoenix, Arizona 85015
602-759-1245
Fax 602-759-8331
Editorial
Our thanks to Jerry Stine, NC, Director of Lifespan Institute for his technical support on this article. (www.Lifespan-Institute.com)
Nancy Faass, MSW, MPH, is a writer and editor in San Francisco who has worked on more than 40 books for publishers that include Elsevier, McGraw-Hill, Mosby, and others. Director of the Writers' Group, she provides articles, white papers, and writing for the Web via HealthWritersGroup.com.

References
1. Cascella NG, et al. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011 Jan;37(1):94-100. Epub 2009 Jun 3.
2. Fasano A, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286-92.
3. Sanders DS, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003 Apr;15(4):407-413.
4. West J, et al. Seroprevalence, correlates and characteristics of undetected coeliac disease in England. Gut. 2003 Jul;52(7):960-965.
5. Hadjivassiliou M, et al. The immunology of gluten sensitivity: beyond the gut. Trends Immunol. 2004 Nov;25(11):578-582.
6. Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001 Feb;120(3):636-651.
7. Ventura A, et.al. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology. 1999 Aug;117(2):297-303.
8. Bonamico M, et al. Tissue transglutaminase autoantibody detection in human saliva: a powerful method for celiac disease screening. J Pediatr. 2004 May;144(5):632-636.
9. Ludvigsson JF, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2012 Feb 16. doi:10.1136/gutjnl-2011-301346.
10. Vojdani A. The characterization of the repertoire of wheat antigens and peptides involved in the humoral immune responses in patients with gluten sensitivity and Crohn's disease. ISRN Allergy. 2011. doi:10.5402/2011/950104.
11. Glicksman E. Catching autism earlier. Monitor Psychol. 2012 Oct;43(9): 57-60.
12. Van den Broeck HC, et al. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: wheat breeding may have contributed to increased prevalence of celiac disease. Theor Appl Genet. 2010 Nov;121(8):1527-1539.
13. Tye-Din JA, et al. Comprehensive quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010 Jul;2:1-14.
14. Vojdani A, Tarash I. Cross-reaction between gliadin and different food and tissue antigens. (Submitted).


|
|



![]()
![]()
![]()


 Aristo Vojdani, PhD, MSc, CLS
Aristo Vojdani, PhD, MSc, CLS