|
Page 1, 2
One of the hallmarks of integrative medicine is to focus on determining the underlying causes of chronic illnesses for patients, not just to treat symptoms. This has led many practitioners of integrative medicine to use testing that helps provide more personalized care protocols to their patients. It is the duty of diagnostic laboratories to deliver the most accurate test results possible and provide interpretations of the results to help practitioners understand the correlations between results and their patients' conditions. At The Great Plains Laboratory, we have been working on finding new ways to detect underlying causes of many illnesses and studying correlations between the results and conditions. Using results from both our MycoTOX Profile as well as our Organic Acids Test (OAT) (both urine tests), we have discovered correlations between them and uncovered new routes of treatment for patients with mold exposure.
 Townsend Letter provides a platform for those examining and reporting on functional and integrative medicine. Please support these independent voices. Townsend Letter provides a platform for those examining and reporting on functional and integrative medicine. Please support these independent voices. |
For several decades, new research has gone into the detection and treatment of mold mycotoxins. These are the toxic metabolites produced by certain types of fungi. These small molecules are often carried on dust particles or food or are present in water-damaged buildings and homes. The most common routes of exposure are inhalation, skin contact, or ingestion through contaminated food.1 Exposure can lead to different types of chronic health conditions depending on the age, sex, genetics, and health status of the patient. Common symptoms of mycotoxin exposure are fatigue, headaches, rashes, food sensitivities, joint pain, and cough.2-4 Mycotoxins can induce disease through different routes such as cytotoxicity, immunosuppression, and DNA damage.
Mycotoxins can cause cytotoxicity by inhibiting multiple pathways within the cell. Mycotoxins inhibit the production of proteins by interfering with the function of the ribosome. Multiple mycotoxins bind to different subunits that are involved in protein biosynthesis, such as the 60S subunit of the ribosome.5,6 In addition to inhibition translation, mycotoxins can also activate Jun N-terminal Kinase (JNK), mitogen-activated protein kinases (MAPKs), and p38.7,8 Activation of these stress response elements in the cell can lead to cellular damage.
Through their mycotoxin metabolites, pathogenic fungi can reduce the efficacy of the immune system by suppressing multiple different components of it. Mycotoxin metabolites can suppress T and B lymphocyte activity, inhibit immunoglobulin production, and reduce antibody production. Some species of fungi release mycotoxins which can interfere with which genes are activated. Some also release polysaccharides which can result in the induction of neutrophil apoptosis. All these processes result in the body's inability to fight off the fungi as well as other infections.9-11
DNA damage caused by mycotoxins has been shown to be carcinogenic.12 There are two different pathways in which mycotoxins can damage DNA. First is the ability for some mycotoxins to covalently bind to DNA nucleotides. Mycotoxins can covalently bind to the N-7 position of guanine and the N-3 position of adenine, both of which can interfere with DNA synthesis. These adducts can cause an incorrect substitution of a nucleotide or cause deletion in the DNA code.13 The second cause of DNA damage from mycotoxins is the inhibition of DNA topoisomerase I and II. These enzymes are required to untangle DNA during replication, and preventing this activity can lead to the accumulation of DNA breaks.14
Types of Mold and Mycotoxins
Mycotoxins are produced by filamentous fungi, which are ubiquitous because of their ability to thrive in many different types of environments.15 The most common mycotoxins are produced from the fungi genera of Aspergillus, Fusarium, Penicillium, and Stachybotrys. Many of fungi species can produce more than one type of mycotoxin (Table 1, .pdf). In this table, we list ten of the most common mycotoxins, and we can depict which mold species produce these mycotoxins. Aspergillus is the most prevalent mold genus in the environment. The two most common Aspergillus mycotoxins are aflatoxin and ochratoxin, and their main target is the liver.16,17 Aspergillus species are commonly associated with indoor air problems.18 The most common route of transmission for Aspergillus is inhalation.19
Fusarium fungi grow best in temperate climate conditions.20 Fusarium is present in both indoor as well as outdoor environments.21 It also grows worldwide on many different types of grains including corn and wheat.22 Most trichothecene mycotoxins are produced from Fusarium species.
Penicillium is a blue-green mold found on fruits, vegetables, and indoor environments. Many different types of citrus fruits can become contaminated with Penicillium, but it can also contaminate seeds and grains. Factors leading to a contamination of Penicillium in the home, work, or school environment include inadequate heating and ventilation,water leaks, and low sunlight.23
Stachybotrys is a greenish-black mold. This mold can grow on materials with high cellulose and low nitrogen content such as gypsum board, paper, fiberboard, and ceiling tiles. The humidity requirements for Stachybotrys are much higher than other fungi, around 93%, whereas other fungi grow at approximately 75%, and it often occurs in the presence of other fungi.3,24 Toxicity caused by Stachybotrys is mostly caused by the toxins and other compounds produced, and less from particle penetration from the spores.
Detection of Mold Exposure
For decades, it has been difficult to determine if patients have been exposed to mold toxins. Research and testing in the agricultural market has proven helpful in testing and treatment methods for humans. One method of detection, which was developed a decade ago, is Enzyme Linked Immunosorbent Assay (ELISA), which has been used to detect mycotoxins in many different types of crops. There are two barriers in transferring this technology to human samples. These barriers are insufficient sensitivity and matrix effect (ME) in human samples. The first barrier is caused by the small amounts of mycotoxins in human blood and urine, which are both much less than seen in crops. The FDA action level for aflatoxin is 20 ppb (20 µg/L), which is a factor 100 times higher than seen in human samples.25,26 Sample ranges for many mycotoxins in humans are normally in the 60-2000 ng/L (.06-2 µg/L) range.26
The matrix effect barrier is caused by compounds in the sample matrix that are not related to the analyte being measured but can cause a result to be either higher or lower than the actual amount. These MEs can influence any type of test, and protocols must be performed in order to separate the analyte of interest from these interferences.27 Studies in the past eight years have focused on a new technology to access mycotoxin exposure, which is liquid chromatography mass spectrometry (LC-MS/MS).
The introduction of LC-MS/MS has allowed scientists and practitioners to quantitatively measure many different chemicals. These measurements can be done on compounds either individually or simultaneously if the compounds have similar molecular characteristics. A mass spectrometer is a device that measures the molecular mass of compounds. Many different fields have used this technology and two of the most common are drug testing and chemical exposure testing, which both need the sensitivity and selectiveness that LC-MS/MS provides.28 Over the last 10 years in the scientific community, LC-MS/MS has become the gold standard for the measurement of mycotoxins.29
At The Great Plains Laboratory, we use two methods of detection to determine the best course of treatment for patients. To detect mycotoxins, we utilize LC-MS/MS technology because of its sensitivity as well as its selectivity in determining analytes. Our laboratory experts have developed a method for extracting mycotoxins out of human matrixes that provides the most sensitive and accurate data available. We recommend that all patients who take our LC-MS/MS test for mycotoxins, also take an Organic Acid Test (OAT), and both tests can be run on the same urine sample. The Organic Acids Test is a metabolic test performed on the gas chromatography mass spectrometer (GC/MS). The OAT provides a snapshot of how the body is performing metabolically and it offers four distinct areas of information that can assist in the treatment of mycotoxin exposure. These areas are fungal markers, glutathione markers, mitochondrial markers, and yeast/clostridia markers.
To better understand how the OAT and mycotoxin test overlap, we ran a study of 100 healthy patients and 150 mycotoxin-exposed patients. These results are depicted in Figure 1. All of these values were statistically significant with p-values below 0.05. The first insights that we observed in patients that took both the mycotoxin test and the OAT was that two markers in the yeast/fungal section of the OAT were statistically elevated over healthy controls. These two markers were 5-hydroxymethyl-2-furioc and Furan-2,5-dicarboxylic, which are both produced by species of Aspergillus (Figure 1).30,31 Elevations in these two markers could indicate that the patient has a colonization of Aspergillus in the body. Aspergillus is the most common fungi to colonize the body. Because Aspergillus spores are smaller than most other species, 2 µm to 10 µm, they can reach the lower airways. This can lead to fungal colonization, which results in prolonged mycotoxin exposure and allergic responses. The most common areas of colonization are the sinus cavities, the lungs, and the gut.32 Our data indicates that 90% of patients with elevations in these two markers also have elevations of mycotoxins. These data have been shown to be extremely beneficial in determining the best treatment of patients.
 The second benefit for using the OAT in combination with the mycotoxin test is to measure detoxification capacity. In Figure 1, we demonstrate that pyroglutamic acid is elevated in mold-exposed patients, which indicates these patients have a higher usage of glutathione (GSH). Detoxification of many mycotoxins is dependent on the endogenous molecule GSH. GSH is a tripeptide, which is made of glycine, cysteine, and glutamate. Mycotoxin toxicity is intensified in patients with glutathione transferase mutations (GSTP1 and GSTM).33 Glutathione status is measured in the OAT with pyroglutamic acid and 2-hydroxybutyric acid. Patients that are exposed to mycotoxins can have elevated amounts of both compounds (Figure 1). High pyroglutamic acid occurs when the body has been depleted of glutathione, which then activates the γ-glutamyl cysteine synthetase, which leads to the production of pyroglutamic acid.34 2-Hydroxybutyric has also been linked to glutathione production. Patients with higher amounts could be glutathione deficient.35 Using this information will allow practitioners to determine what dose of glutathione would be most appropriate for a patient. The second benefit for using the OAT in combination with the mycotoxin test is to measure detoxification capacity. In Figure 1, we demonstrate that pyroglutamic acid is elevated in mold-exposed patients, which indicates these patients have a higher usage of glutathione (GSH). Detoxification of many mycotoxins is dependent on the endogenous molecule GSH. GSH is a tripeptide, which is made of glycine, cysteine, and glutamate. Mycotoxin toxicity is intensified in patients with glutathione transferase mutations (GSTP1 and GSTM).33 Glutathione status is measured in the OAT with pyroglutamic acid and 2-hydroxybutyric acid. Patients that are exposed to mycotoxins can have elevated amounts of both compounds (Figure 1). High pyroglutamic acid occurs when the body has been depleted of glutathione, which then activates the γ-glutamyl cysteine synthetase, which leads to the production of pyroglutamic acid.34 2-Hydroxybutyric has also been linked to glutathione production. Patients with higher amounts could be glutathione deficient.35 Using this information will allow practitioners to determine what dose of glutathione would be most appropriate for a patient.
Figure 1
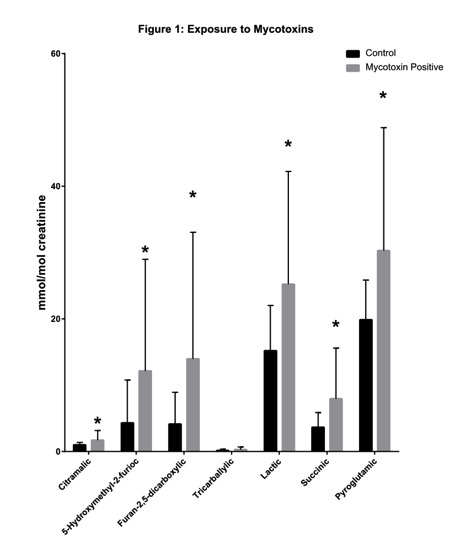
The third benefit of using the OAT for patients is assessing how the mold mycotoxins are affecting the mitochondrial pathways in the patient's body. Many different types of mycotoxins can bind with ribosomes in the cell as well as ribosomes in the mitochondria. These interactions can inhibit proper cellular functions.6 The OAT assesses multiple different mitochondrial pathways. Studies done by The Great Plains Laboratory have demonstrated that patients who have been exposed to mold toxins accumulate lactic acid and succinic acid in their urine (Figure 1).
Page 1, 2
|
![]()
![]()
![]()
![]()







