The purpose of this article is to provide the practitioner with ammunition
to support the use of provocation testing for toxic element burden,
and to increase awareness and stimulate thought about current "hot
topics" regarding the testing process and interpretation of the
test results. Discussion will be limited to the thoroughly researched
pharmaceutical compounds, Ca-EDTA, DMSA, and DMPS. It is emphasized
that the material is presented from the laboratory perspective by a
biochemist/pre-clinical research pharmacologist, not by a practicing
clinician.
Basic Toxicology in a Modern, Preventive Context
The long-standing, basic logic of toxicology dictates there must first be exposure,
then assimilation and net retention of a toxin before one can make valid
conclusions about toxicity in an individual. Classically, this pertained
to acute poisoning, but it has become increasingly accepted that sub-clinical
metal toxicity (SCMT) exists and is typically a consequence of chronic low-level
or intermittent exposure to toxic metals. However, it is generally not accepted
that SCMT requires clinical intervention. Clearly, the term "sub-clinical
toxicity" is a bit of an oxymoron, and the phrase actually relates
to sub-threshold toxicity, which simply means that the level of retention
of the toxin has not been established to be associated with overt poisoning
in the vast majority of individuals. The operative word here is individuals,
a concept that, to date, only seems to be much appreciated by those who subscribe
to and practice preventive/complementary/functional medicine.
The basic model of toxicology is quite logical, but needs to be applied in
an updated, preventive context as opposed to crisis management. One must concede
to the fact that exposure alone should not be used to make diagnostic decisions
about chronic toxicity, but rather a quantitative assessment of net retention
of metals provides the clinician with objective, arguable data. Importantly,
net retention is determined by the difference between the rates of assimilation
and irreversible excretion of a toxin. The idea that a set threshold value
for metal retention is associated with toxicity may be applied to large-scale
population studies, but it is clear that there is tremendous variability among
individuals with respect to physiological "tolerance" to retained
metals. In reality, for a given individual, toxicity is exhibited when the
level of net retention exceeds physiological tolerance. Such individual tolerance,
and the capacity to excrete metals by means of endogenous, inducible processes,
is affected by one's genetically based capacity to express specific proteins
(e.g., metallothionine, glutathione), nutritional status, antibiotic use, lifestyle,
and total toxic load (all metals, organic xenobiotics, pharmaceutical and recreational
drugs, and gut-derived toxins).
Provocation Testing: Validation and Essential Considerations
Currently, the best approach to assessing the net retention or body burden
of toxic metals is urinalysis after administration of well-established chelators
or metal-binding agents such as EDTA, DMPS, or DMSA. Strong support for this
approach is provided by a recent statement included in a new draft available
for public comment on the Agency for Toxic Substances and Disease Registry
(ATSDR) website:1
The measurement of lead excreted in urine
following an injection (intravenous or intramuscular) of the chelating
agent calcium disodium EDTA
(EDTA provocation)
has been used to detect elevated body burden of lead in adults2-5 and children,6,7
and is considered to be a reliable measure of the potentially toxic fraction
of the lead body burden.8
Further, the relationship between blood
lead and post-EDTA urinary lead is non-linear, in that arithmetic increases
in blood lead are associated with
EXPONENTIAL increases in urinary lead-EDTA complexes.9 So, the precedent
has clearly been set, and it follows logically that one can assess the
body
burden
of lead, mercury, arsenic, cadmium, and other toxic metals using other
validated agents that have similar pharmacological mechanisms of action,
albeit with
very different affinities for specific metals. An extensive review of the
affinities and clinical utilities of Ca-EDTA, DMSA, and DMPS has recently
been presented.10
To make valid conclusions about body burden utilizing provocative challenges,
it is imperative to have objective data to permit distinction between recent
exposure to metals vs. that which has been retained by tissues and is not
simply in circulation. Such data are obtained by comparison of urinary
metal levels
in a pre-provocation urine specimen (very recent exposure) and that following
administration of a chelator/metal-binding agent. Ideally the pre- and
post-specimens should be collected in the closest possible proximity. It
is recommended
that the pre-provocation specimen be collected as the first morning void
on the
same day as the challenge test. The most commonly utilized challenge protocols
entail a complete six-hour collection after intravenous (IV), oral, or
rectal administration of an agent. Hence, the two required specimens could
easily
be collected in the same day. Based on a comparison of the pre- and post-urinary
metals DATA, one can formulate a professional opinion about the potential
adverse health effects of a patient's retention of toxic metals. It is
very likely
that, in most cases, with the exception of organic arsenic derived from
consumption of shellfish within about 48 hours of the collection, pre-provocative
urine
specimens contain very low levels of toxic metals. However, in the event
of a challenge by legal/medical adversaries, if one does not have the complete
set of data to discriminate between recent exposure vs. net retention,
the outcome is likely not favorable for the practitioner. Due to such litigation
and overwhelming legal fees, one doctor has recently adopted a strict policy
to no longer work with patients who refuse to submit to initial pre- and
post-provocation
urinary metals tests. As with all laboratory analyses, the results must
be
considered in context with the patient's history, symptoms, and other
laboratory tests results. It should be kept in mind that the aforementioned
provocative agents do not appreciably cross a healthy blood brain barrier
and are too hydrophilic to provide direct information about metals retained
in
the lipid-rich CNS. Facilitation of Maximal Yields
Antioxidants
Many suggestions have been made towards maximizing urinary metal yields post-provocation,
such as co-administration of reduced glutathione and other natural compounds/nutrients,
but convincing validation of efficacy is lacking from the laboratory point
of view. However, there are a couple of exceptions that should be considered.
Numerous peer-reviewed papers have recently been published that conclusively
indicate that co-administration of antioxidants such as N-AC, alpha-lipoic
acid, melatonin, and vitamins E and C improve DMSA-induced lead detoxification.11-13
The beneficial effects are not due to direct binding and excretion of lead
by the antioxidants, but rather due to associated improvement of the cellular
redox state and amelioration of oxidative stress and damage that enables enhanced
endogenous detoxification. The aforementioned studies did not address acute
provocation testing, but rather long-term efficacy of detoxification therapy.
Thus, it is not implicitly implied that acute co-administration of the antioxidants
will significantly increase provocative yields. The studies do provide strong
support for the use of appropriate antioxidant supplementation prior to provocation
testing and throughout a comprehensive metal detoxification regime.
L-glycine
L-glycine is a direct assisting agent for increasing post-provocative
urinary metals.14 In contrast to the effects of the antioxidants
mentioned above,
acute administration of L-glycine can markedly increase the urinary spill
of toxic metals when used in conjunction with Ca-EDTA, DMSA, and DMPS.
As a naturally occurring amino acid, L-glycine, unlike the synthetic
agents,
readily crosses cell membranes (two-way street). Having a relatively weak
but functionally significant affinity for metals such as mercury, aluminum,
nickel, lead, and antimony, L-glycine can facilitate the movement of metals
from within cells to the extracellular compartment where the pharmaceutical
agents are restricted. With higher affinities for the glycine-mobilized
intracellular metals, the circulating metal-binding agents preferentially
snatch the metals
like alpha dogs and carry them to the kidneys for irreversible excretion
in urine. L-glycine is particularly useful for enhancing EDTA-induced removal
of aluminum.14 Unpublished observations at DDI indicate that L-glycine
also increases the excretion of lead, mercury, and antimony when
used orally in
conjunction with DMSA and DMPS. Practitioners who have used the assisting
agent are ecstatic with the increased yields. It was previously recommended15
that oral L-glycine be administered both the day before a provocation (80
mg/kg in divided doses) and in the morning on the day of an EDTA challenge
(40 mg/kg about two hours before IV administration of EDTA). A more conservative
protocol is recommended for safety sake when L-glycine is used in conjunction
with DMSA or DMPS – binding agents that have essentially no affinity
for aluminum. Since L-glycine so effectively mobilizes aluminum, and the
dithiol compounds do not subsequently bind aluminum, the EDTA-associated
L-glycine protocol could result in unintended redistribution of aluminum
to more vulnerable cells such as neurons (bad ping-pong effect). Hence, when
utilizing L-glycine as a potent assisting agent with DMSA or DMPS, it seems
prudent to reduce the L-glycine dose to 40 mg/kg (orally) about two hours
prior to administration of the dithiol agent of choice.
The L-glycine boost may be especially helpful for obtaining higher metal
spills for "sensitive" patients for whom one might anticipate problems
with the most productive DMSA challenge of 30 mg/kg, oral bolus not to exceed
2 gm.16 In such cases, doctors have gotten impressive results using just a
single dose of 10 mg/kg DMSA combined with the latter protocol for L-glycine,
followed by a six-hour urine collection. Otherwise, the low-dose DMSA challenge
is significantly less productive and may be of limited clinical value.17
Due to the capacity of L-glycine to mobilize and potentially cause redistribution
of metals, especially aluminum, it is emphasized that the assisting agent
should not be used alone. Glycine also has the potential to increase assimilation
of dietary metals and is contraindicated for patients with hyperammonemia,
abnormally elevated plasma glycine, and/or serine, and those suspected of
or
diagnosed with schizophrenia or other psychoses.15 The use of L-glycine as
an assisting agent for provocations appears to be quite safe when used as
described. However, as tempting as it is after one sees such improved provocation
results,
the safety of long-term use of L-glycine supplementation as a component of
a sustained metal detoxification protocol has not been established. Potential
concerns include excessive production of oxalic acids and exacerbation of
disorders of methionine metabolism, e.g., methylation defects.15 DMSA Suppositories
DMSA is the active compound in an FDA-approved product (Chemet®) for
lead detoxification. DMSA has also been well-established as an enhancement
for urinary excretion of mercury, antimony, and, to a lesser extent, some other
metals. DMSA cannot be given intravenously, and when used orally, it can be
associated with exacerbation of gastrointestinal dysbiosis and distress and
even exacerbation of symptoms in autistic children.18 Particularly
as a result of the observed adverse effects in Autism Spectrum Disorders (ASD)
children,
there has been increased interest and use of rectal suppositories of DMSA as
a para-oral delivery system. Provocation tests using oral DMSA can be very
challenging for parents, and intravenous use of Ca-EDTA or DMPS is not always
a viable option. Therefore, a study was conducted to determine if clinically
useful information about metal retention could be obtained from rectal administration
of DMSA followed by a six-hour urine collection.19 The subjects
were five autistic children (three to four years old), who had never received
any treatments or
provocative tests for metal retention. The dose DMSA in the suppositories was
20 mg/kg (none > 500 mg), and no adverse effects were reported. Early morning
urine voids were collected on the day of provocation and served as the pre-specimens.
Comparison of pre- and post-provocative urinary metals revealed significantly
increased excretion of lead and mercury in all five children. There were marginal
effects on nickel excretion, and no consistent increases were observed for
any other metal for these particular children. Figures
1 and 2 (below) clearly illustrate
the consistency and magnitude of the effects of DMSA suppositories on acute
excretion of lead and mercury, respectively. These exciting results provide
direct evidence that rectally administered DMSA can effectively increase the
elimination of retained metals, and the suppository route offers another option
for provocation testing in this population. Although not yet as strictly evaluated,
test results from DDI indicate that similar effects are likely to be obtained
with rectally administered DMPS suppositories.19
Figure 1
DMSA suppositories and acute increase in urinary
lead excretion in autistic children. For
each of the five children, paired pre- and post-provocation
lead levels are presented as the striped and solid bars, respectively. Urinary
lead levels are expressed as µg lead/gm
creatinine.
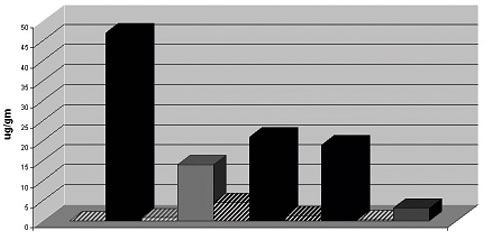
Patient Pre vs. Post
We have analyzed urinary metals from 35 adults before and after
rectal administration of a proprietary Ca-EDTA suppository (750 mg Ca-EDTA)
and did not detect a
significant acute effect on metal excretion. However, by comparison of
post-DMSA urinary metals before and after ninety days of nightly administration
of the
suppositories in the same subjects, significant reductions were observed
for specific metals. Strictly from a laboratory perspective, the Ca-EDTA
suppository
data indicate that at the dose utilized (750 mg), the suppositories did
not yield urine metal spills that compare to that of intravenous Ca-EDTA
or DMPS,
oral DMSA or DMPS, or DMSA suppositories. However, the Ca-EDTA suppositories
appeared to show efficacy in the long run. Several new studies regarding
the same Ca-EDTA suppositories are allegedly forthcoming. (In
this issue of the
Townsend Letter,
Dr. Garry Gordon presents data regarding the clinical utility of orally
administered Ca-EDTA.)
Figure 2
DMSA suppositories and acute increase in urinary
mercury excretion in autistic children. For
each of the five children, paired pre- and post-provocation
lead levels are presented as the striped and sold bars, respectively. Urinary
mercury levels are expressed as µg
mercury/gm creatinine.
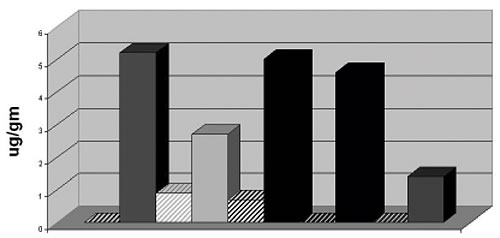
Patient Pre vs. Post
Urinary Lead/gm Creatinine in Middle-Aged Women
Peri- and postmenopausal woman constitute a sub-population of patients for
whom much concern has been raised with respect to "re-infusion" of
lead from vast bone stores to soft tissues. Recent studies have indicted
that such women are at significantly increased risk for cardiovascular disease
(CVD), cardiovascular mortality,20 and both systolic and diastolic hypertension.21
If one is short on reading material, a literature search including key words
such as lead, menopause, estrogen, and osteoporosis will provide a plethora
of research that has been conducted in this area. The problem, however, is
that although the concern is real, little mention is made regarding therapeutic
intervention short of high-priced pharmaceuticals that have horrific side
effects.
The problem with lead for this particular group is obviously the increased
turnover of the bone matrix – in large part, a result of decreased estrogen.
As about 95% of lead is stored in bone in adults, the hormonal change is associated
with a new, increased rate of release of lead from bone to soft tissue. Although
hardly benign in bone, lead in the central nervous system (CNS), immune system,
kidneys, and the arterial endothelium is of much greater concern. From a laboratory
perspective, concern has been expressed by some physicians regarding the expression
of post-provocation urinary lead as a function of urinary creatinine (e.g., µg
lead/gram creatinine). Specifically, the suggestion has been put forth that
since many woman in this phase of life often have low levels of lean body mass
(muscle) and associated low levels of creatinine, their lead levels as reported
are "inflated." This concept has raised uncertainty for some as
to the clinical significance of the test results.
Let's think about this systematically. First, most post-provocation urinary
creatinine levels, expressed as mg/dL, are on the low end. It must be understood
that urinary creatinine concentrations in a spot or timed urine collection
provide no clinically relevant information when considered alone, because the
creatinine is diluted in urine as a function of urine volume. Patients are
instructed to consume adequate amounts of clean water/fluids after receiving
a provocative agent to ensure good flushing of the kidneys. To the same extent,
the mobilized lead is ALSO diluted, hence the standardization of lead per creatinine
to eliminate the confounding factor of variable urine volume. To determine
if creatinine production is low, if, in fact, glomerular filtration is normal,
it is easy to measure serum creatinine. One can also physically evaluate musculature.
For the sake of discussion, let's assume that glomerular filtration is
normal, and serum creatinine and total 24-hour creatinine excretion are both
low as a function of low musculature. Compared to an athletic or physically
fit patient of the same gender and age, with the same absolute amount of lead
mobilized by DMSA or EDTA, the patient with the low creatinine excretion will
appear to have a higher lead burden than the other (greater lead/gm creatinine).
One interpretation might be that the "frail" patient doesn't
really have a lead issue; she just has lower creatinine. An alternative interpretation
is that since the patient with the lower creatinine has less lean body mass,
which might be envisioned as a sort of buffer, her kidneys, vascular endothelium,
spleen, liver, and especially her CNS might be more likely to accumulate that
lead and incur a greater degree of lead-induced adverse effects. Although muscle
tissue is not generally considered to be a major depot for lead, consider the
amount of calcium in muscle that is required for muscle contraction. Lead is
very similar to calcium at the elemental/atomic level, and lead "follows" calcium
metabolism in the body. All things considered, it seems logical to conclude
that in such a case the patient with the greater amount of retained lead per
gram/creatinine might be at greater risk for adverse health effects of lead.
What say you?
Manganism and Parkinson's-Like Disease
Manganese (Mn) is an essential element for which homeostasis is maintained
by tight regulation of oral assimilation (about one to three percent) and efficient
excretion in the bile by a healthy liver. However, when retained in excess,
Mn can become extremely neurotoxic. The clinical manifestations of manganism
pertain to extrapyrimidal syndrome in a pattern similar to but not identical
to Parkinson's disease.22,23 Most prevalent are intentional tremor with
absent or low level of resting tremor, hypertonia, gait disturbance (particularly
difficulty walking backwards), apathy, poor cognitive function and memory,
and even psychosis.24,25 Manganism is most commonly associated with occupational
exposure, primarily due to particulate/vapor uptake of Mn by the lungs. However,
manganism can also result from liver or biliary disease. Although manganism
has been well-documented and studied, clinical intervention has been largely
unsuccessful, especially if not detected in early stages.
Excessive Mn retention can be readily detected by comparison of urinary Mn
levels before and after intravenous injection of Ca-EDTA. It is absolutely
critical to measure basal urinary Mn, because EDTA has a relatively high affinity
for Mn. In a study of fourteen healthy medical personnel from a medical clinic
in Southern California, urinary Mn was increased 15-X (average) over baseline
after a three gm IV push of Ca-EDTA.26 Recently, a very astute practitioner
with many years of experience in metal detoxification suspected Mn toxicity
in a patient whose Parkinson's-like symptoms improved transiently after
intravenous EDTA treatment. Urinalysis for toxic metals was unremarkable, so
it was recommended that he do a pre- and post-Ca-EDTA urinalysis for essential
elements (e.g., Mn, iron, copper). Basal urinary Mn was within normal range,
but urinary Mn after Ca-EDTA increased 300 times. EDTA has been associated
with only transient improvements in Mn-induced neurological symptoms at best.
However, it has recently been reported that extensive intravenous treatment
with para-aminosalicylic acid (PAS) resulted in near complete and sustained
(17-year) resolution of Mn-induced neurological symptoms in a patient who previously
had extreme, prolonged occupational exposure to Mn.27 PAS is an antibiotic
that has anti-inflammatory properties, and it has been reported to increase
fecal and urinary excretion of Mn in rabbits.28 Additional basic research and
clinical trials seem to be warranted regarding PAS-induced Mn excretion, as
well as the potential role of PAS in the treatment of other neurological diseases
such as Parkinson's and Alzheimer's diseases.
Anticipation is high for the outcome for the patient with Parkinsonianism who
had a post-Ca-EDTA urinary Mn excretion 20 times greater than expected. This
case clearly illustrates the power and clinical utility of provocation testing
for the assessment of excess net retention of toxic metals and potentially
toxic elements. Had the doctor not properly performed the challenge test, the
likely root cause of the patient's neurological disorders would have
remained a mystery.

David W. Quig, PhD
Vice President, Scientific Support
Doctor's Data, Inc.
800-323-2784
inquiries@doctorsdata.com

Notes
1. Agency for Toxic Substances and Disease Registry. Toxicological
profile. Available at: www.atsdr.cdc.gov/toxprofiles/tp13.html#. Accessed
April 11, 2007.
2. Biagini G, et al. Renal morphological and functional modification in chronic
lead poisoning. In: Brown SS, ed. Clinical Chemistry and Chemical Toxicology
of Metals. Amsterdam: Elsevier/North-Holland
Biomedical Press; 1977:123-126.
3. Lilis RM, et al. Nephropathy in chronic lead poisoning. Br J Ind Med. 1968;25:196-202.
4. Wedeen RP, et al. Occupational lead nephropathy. Am J Med. 1975;
59:630-641.
5. Wedeen, RP. Removing lead from bone: Clinical implications of bone lead stores.
Neurotoxicol. 1992; 13:843-852.
6. Chisolm J, et al. Interrelationships among blood lead concentration, quantitative
daily ALA-U and urinary lead output following calcium EDTA. In:Nordberg GF, ed.
Proceedings of Third Meeting of the Subcommittee on the Toxicology of
Metals
Under the Permanent Commission and International Association on Occupational
Health, November 1974, Tokyo, Japan. Amsterdam,
Netherlands: Elsevier Publishing
Co.; 1976: 416-433.
7. Markowitz Meet, et al. Zinc (Zn) and copper (Cu) metabolism in CaNa2 EDTA-treated
children with plumbism. Pediatr Res. 1981;15:635.
8. WHO. Environmental transport, distribution and transformation. Geneva, Switzerland:
World Health Organization; 1995:60-65.
9. Goyer RE, et al. Role of chelating agentsfor prevention, intervention, and
treatment of exposures to toxic metals. Environ Health Perspect. 1995;103:
1048-52.
10. Quig DW. Chronic metal toxicity: assessment of exposure and retention. In
Textbook of Natural Medicine. 3rd edition.
J.E.Pizzorno, Jr, ed. Amsterdam: Elsevier;
2004:263-74.
11. Flora SJ, et al. Beneficial effect of combined administration of some naturally
occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic
lead intoxication. Chem Biol Interact.
2003;15:267-80.
12. Flora SJ, et al. Lead-induced oxidative stress and its recovery following
co-administration of melatonin or N-acetylcysteine during chelation with succimer
in male rats. Cell Mol Biol. 2004;50.
13. Pande M, et al. Lead induced oxidative damage and its response to combined
administration of alpha-lipoic acid and succimers in rats. Toxicol. 2002;177,187-96.
14. Garrot P. Metabolism and possible health effects of aluminum. Environ
Health
Perspect. 1986; 65:363-411.
15. Pangborn JB. In: Mechanisms of Detoxification and Procedures for Detoxification. St.
Charles, Illinois: Doctor's Data, Inc. and Bionostics, Inc.; 1994:123-5.
16. Hibberd AR, et al. Mercury from dental amalgam fillings: studies on oral
chelating agents for assessing and reducing mercury burdens in humans. J
Nutr
Environ Med. 1997; 8:219-31
17. Quig DW. Unpublished observations. Doctor's Data, Inc.; 2002.
18. Autism Research Institute, Think Tank participants. Unpublished observations.
2002.
19. Quig DW. The efficacy of rectal suppositories containing dithiol metal binding
agents for assessment of metal retention in autistic children (in preparation).
20. Lustberg M, et al. Blood lead levels and mortality. Arch Intern Med.
2002;162:2443-49.
21. Nash D, et al. Blood lead, blood pressure and hypertension in perimenopausal
and postmenopausal women. JAMA. 2003;289:1523-32.
22. Olanow CW. Manganese-induced parkinsonianism and Parkinson's disease.
Ann NY Acad Sci. 2004;1012:209-23.
23. Pal PK, et al. Manganese Neurotoxicity: a review of clinical features, imaging
and pathology. Neurotoxicol. 1999;20:227-38.
24. Wang JD, et al. Manganese-induced parkinsonism: an outbreak due to an unrepaired
ventilation control system in a ferromanganese smelter. Br J Ind Med. 1989;
46:856-59.
25. Josephs KA, et al. Neurological manifestations in welders with palladial
MRI T1 hyperintensity. Neurology. 2005;
64:2033-39.
26. Quig, DW, et al. The acute effects of fast-push Ca-Na2-EDTA on biliary/fecal
and urinary excretion of toxic and essential elements. (In preparation)
27. Yue-Ming J, et al. Effective treatment of manganese-induced occupational
parkinsonism with r-aminosalicylic acid: A case of 17-year follow-up study. J
Occup Environ Med. 2006;48:644-49.
28. Tendon SK. Chelation in metal intoxication. IV. Influences of PAS and CDTA
on the excretion of manganese in rabbits given MnO2-. Toxocology. 1978:9:379-85.
|



![]()
![]()



