Page 1, 2
What Can Cause Neurotransmitter Deficiency or Imbalance Neuropsychiatric Lyme?
Lyme disease can disrupt the neurotransmitters' circuitry throughout the central nervous system.13 One of the main factors that aggravate circuitry imbalances in Lyme disease or Lyme neuroborreliosis is mitochondrial dysfunction. Damage to the mitochondrial electron transport chain has been suggested as an important factor in the pathogenesis of a range of psychiatric disorders. Tissues with high energy demands, such as the brain and the heart, contain a large number of mitochondria. These very active mitochondria-dense organs are more susceptible to reduction of the aerobic metabolism. Internal and external stressors, including infections, pose a higher metabolic demand on the mitochondria to produce the necessary energy to maintain homeostasis. When the allostatic load becomes extreme, the energy demands increase exponentially, taxing the mitochondria and resulting in mitochondrial dysfunction, which creates inefficient energy production and leads to energy deficit. This in turn creates a disparity between very high metabolic energy demands in inflammatory states and the actual energy deficit resulting from mitochondrial dysfunction. Mitochondrial dysfunction and inefficient energy production compromise normal metabolic function, including neurotransmitters and hormones synthesis, metabolism, and detoxification.
These mitochondrial deregulations result from alterations in the biochemical cascade that damage the mitochondrial electron transport chain. This inefficient energy production is an important factor in the pathogenesis of a range of neuropsychiatric disorders, such as addiction, alcoholism, bipolar disorder, depression, and schizophrenia.21-23 In addition to their crucial role in generation of adenosine 5¢-triphosphate (ATP), mitochondria are involved in other important processes, such as regulation of free radicals, neurotransmitters, calcium, and apoptosis. They are also involved in neuronal development – synaptogenesis, synaptic development, and plasticity. Impaired function of mitochondria leads to impaired bioenergetics, decrease of ATP production, impaired calcium homeostasis, increased production of free radicals, and oxidative stress. Nicotinamide adenine dinucleotide (NAD) deficiency is an integral part of mitochondriopathy.36,38-40 Nicotinamide adenine dinucleotide (NAD+) is a coenzyme found in all living cells.45,46 The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. In metabolism, NAD+ is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is therefore found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD+. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD+ metabolism are targets for drug discovery.
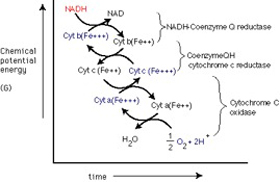
In organisms, NAD+ can be synthesized from simple building blocks (de novo) from the amino acids tryptophan or aspartic acid. Alternatively, more complex components of the coenzymes are taken up from food as niacin.32,33,37 Similar compounds are released by reactions that break down the structure of NAD+. These preformed components then pass through a salvage pathway that recycles them back into the active form. This energy is used for all body functions and activities. It stimulates production of dopamine, serotonin, and noradrenaline, thereby improving mood. A deficiency of this essential fuel of cells is recognized to be the key feature for causing chronic fatigue, persistent inflammation, chronic pain, low energy states, depression, anxiety and alcohol or drug addiction. Medical research has shown that supplementation with NAD results in quick recuperation from infections, cancer treatment, and addiction recovery with no cravings.24,28,31
Hope for Recovery from Lyme Neuroborreliosis Spectrum of Diseases – Including Addiction
Neuroendocrine immune restoration is the mainstay of treatment of Lyme neuroborreliosis and associated neurodegenerative diseases, including addiction. Neuroendocrine immune restoration can only be achieved through repair of the cellular energy centers. We will propose several therapeutic modalities that will simultaneously repair the mitochondriopathies associated with Lyme disease and its concomitant addiction disorders.34,41 Lyme disease management and addiction recovery are possible if NAD levels in the cells are restored to normal concentrations. Several specially crafted intravenous protocols have been established to reenergize the mitochondria. An induction phase includes a tailored blend of specific amino acids combination followed by active replacement of DPN, vitamins, and minerals necessary for reestablishing mitochondrial vitality. Nevertheless, these regimens can be given orally; it may take much longer for recovery because the effectiveness of these building blocks depends on a healthy digestion and absorption of these nutrients. When given intravenously, these elements are immediately available to all cells. In an attempt to directly manage mitochondrial dysfunction and increase energy production, the proper therapeutic approach should include in addition to the intravenous arm an aggressive restoration of B vitamin stores, since some B vitamins (riboflavin, or B2; niacin, or B3) are used to generate molecules that donate electrons to the mitochondrial respiratory chain.44 Other B vitamins (thiamine, or B1) are cofactors in biochemical reactions that generate bioenergetic pathway intermediates such as acetyl CoA. Thiamine, lipoic acid, and dichloroacetate have been used to enhance pyruvate dehydrogenase activity and increase acetyl CoA levels. Molecules capable of accepting electrons from or donating electrons to the respiratory chain (coenzyme Q, idebenone, vitamin K derivatives, methylene blue) are used with the intent of augmenting reduced electron transport through the respiratory chain.
Other approaches for enhancing cell energy supplies have been considered. Using acetyl donors such as acetylcarnitine to increase Krebs cycle carbon flux is one approach. Using the ketone body betahydroxybutyrate to encourage carbon entry into the Krebs cycle is another. More indirect ways of buttressing cell energy reserves include administering compounds such as creatine that can store high-energy phosphate bonds. Furthermore, because defective mitochondria produce elevated amounts of reactive oxygen species (ROS), which causes oxidative stress, antioxidants have also been advocated for the treatment of mitochondrial dysfunction. Natural substances used for their antioxidant potential include intravenous vitamin C, vitamin E, and b-carotene, along with several of the electron acceptors such as coenzyme Q
Conclusion
It is reasonable to consider Lyme neuroborreliosis and its linked neurodegenerative diseases, including addiction, as neurodegenerative mitochondriopathies. The search for effective therapies should always include treatments that target mitochondrial pathways affected. Whether mitochondrial dysfunction in a particular disease is the initiating, intermediate, or downstream factor in neurodegenerative cascades, it requires special considerations.
Page 1, 2

Notes
1. Miklossy J. Alzheimer's disease – a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation. 2011;8:90.
2. Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290.
3. Ling VJ, Lester D, Mortensen PB, Langenberg PW, Postolache TT. Toxoplasma gondii seropositivity and suicide rates in women. J Nerv Ment Dis. 2012;199:440–444.
4. Mortensen CR, Becker DV, Ackerman JM, Neuberg SL, Kenrick DT. Infection breeds reticence: the effects of disease salience on self-perceptions of personality and behavioral avoidance tendencies. Psychol Sci. 2010;21:440–447.
5. Bransfield RC. Preventable cases of autism: relationship between chronic infectious diseases and neurological outcome. Ped Health. 2009;3:125–140.
6. Bransfield RC, Wulfman JS, Harvey WT, Usman AI. The association between tick-borne infections, Lyme borreliosis and autism spectrum disorders. Med Hypotheses. 2008;70:967–974.
7. Träskman-Bendz L, Westling S. The psychobiology of aggressive behaviour. Adv Health Econ Health Serv Res. 2005;16:3–14.
8. Knuesel I. Prenatal infection as driving force of aging-associated neurodegenerative diseases. Praxis (Bern 1994). 2011;100:299–304.
9. Daneman R, Rescigno M. The gut immune barrier and the blood-brain barrier: are they so different? Immunity. 2009;31:722–735.
10. Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J Neurosci. 2010;30:13254–13264.
11. Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol Lett. 2010;198:182–190.
12. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87.
13. Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43.
14. Kristensson K. Microbes' roadmap to neurons. Nat Rev Neurosci. 2011;12:345–357.
15. Bransfield R. Neuropsychiatric Lyme disease: pathophysiology, assessment and treatment. 2nd International Lyme and Associated Diseases Society European meeting; May 2011; Augsburg, Germany.
16. Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11:28–35.
17. Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7:1271–1285.
18. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966.
19. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057.
20. Hong BA, North CS, Pollio DE, et al. The use of psychoeducation for a patient with hepatitis C and psychiatric illness in preparation for antiviral therapy: a case report and discussion. J Clin Psychol Med Settings. 2011;18:99–107.
21. Greenberg HE, Ney G, Scharf SM, Ravdin L, Hilton E. Sleep quality in Lyme disease. Sleep. 1995;18:912–916.
22. Moldofsky H. Sleep and the immune system. Int J Immunopharmacol. 1995;17:649–654.
23. Lorton D, Lubahn CL, Estus C, et al. Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation. 2006;13:357–374.
24. Anokhina IP. Effect of acute and chronic ethanol exposure on rat brain opiate receptor function. Alcohol Alcohol. 1982;18:31–34.
25. Beer JH, Quastel CT. The effect of aliphotic aldehydes on the respiration of rat brain cortex slices. Can J Biochem Physiol. 1958;36:531–541.
26. Cleary JP. Etiology and biological treatment of alcohol addiction. J Neuro Ortho Med Surg. 1985;6:75–77.
27. Davis V, Walsh MJ. Alcohol, amines and alkaloids, a possible biochemical basis for alcohol addiction. Science. Feb. 13, 1970;167:1005–1007.
28. Derosa G. Endocrine study of anorexia nervosa. Exp Clin Endocrinol. 1983;82.
29. Dewan JG. An alcohol detoxification mechanism in the central nervous system. Am J Psych. 1943;99;565–568.
30. Eaton S. Paleolithic nutrition. N Eng J Med. 1985;312(5).
31. Eriksson CJ. Increase in hepatic NAD level – its effect on redox state and on ethanol and acetaldehyde metabolism. FEBS Lett. 1974;40:317–320.
32. Evans VL. Pellagra with psychosis and minimal physical symptoms. JAMA. Apr. 1, 1939.
33. Frostig J, Spies TD. The initial nervous syndrome of pellagra and associated deficiency diseases. Am J Med Sci. Feb. 1940;199.
34. Fiennes RN, Sinclair AJ, Crawford MA. Essential fatty acid studies in primates: linolenic acid requirements of capuchins. J Med Prim. 1973;2:155–169.
35. Hekimian LJ. Treatment of acute brain syndrome from alcohol with nicotinamide adenine dinucleotide and methamenodiazepoxide. Q J Stud Alcohol. 1966;27.
36. Hoffer A, Osmond H, Smythies J. Schizophrenia: a new approach II. Results of a year's research. J Ment Sci. 1954;100:29–45.
37. Kohn H. The concentration of co-enzymelike substances in blood following the administration of nicotinic acid to normal subjects. Biol Chem J. 1938;32:2075.
38. Levine AS. Alcohol and the opiate receptor. Alcohol Clin Exp Res. 1983;7:83–84.
39. Lieber CS. Changes in blood ethanol consumption and acetaldehyde after chronic ethanol consumption. Int Titisee Symp Black Forest Ger. January 1982.
40. Lyon LJ, Anthony J. Reversal of alcoholic coma by naloxone. Ann Intern Med., 1982;96(4):464–465.
41. Mainzer F, Krause M. Nicotinic acid in the treatment of delirium tremens. Br Med J. Aug. 12, 1939;331.
42. O'Holleran P. DPN in the prevention, diagnosis, and treatment of drug addictions. West J Surg Obst Gyn. 1961;69:213–215.
43. Petrie WM. The use of nicotinic acid and pyridoxine in the treatment of schizophrenia. Int Pharmacopsychiatry. 1981;16:245–250.
44. Richards CD. Nicotinamide adenine dinucleotide depresses synaptic transmission in the hippocampus and has specific binding sites on the synaptic membranes. Br J Pharmcol. 1983;79.
45. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16:837–850.
46. Smith RF. Status report concerning the use of megadose nicotinic acid in alcoholics. Orthomol Psych. 1978;7(1).
47. Spies TD, Bean WB. The role of nicotinic acid in the prevention of pellagra, roentgen sickness and increased porphyrinuria. J Clin Invest. 1938;17.
48. Spies TD, Gross ES, Sasaki Y. Effect of yeast and nicotinic acid on porphyrinuria. Proc Soc Exper Biol Med. 1938;38:178–181.
49. Sutton D C Interrelation between the vitamin B complex and the anterior lobe of the pituitary gland. J Lab Clin Med. 1940;25(11).
50. Taborkoff B, Hoffman P. Alcohol interactions with brain opiate receptors. Life Sci. 1983;32:197–204.
51. Vilter RW, Vilter SP, Spies TD. Determination of codehydrogenases I and II (cozymase) in blood of diabetics in severe acidosis. Am J M Sci. Mar. 1939;197:322–326.
52. Vilter SP. Coenzymes I and II in human blood. J Lab Clin Med. 1940;26:31.
53. Yamada K. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. Diabetes. 1982;31:749–753.
54. Yamamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. Nov. 19, 1981;294.
 Dalal Akoury, MD, is the founder of AWAREmed Health and Wellness Resource Center and the director of the Wellness U program. Dr. Akoury is board certified in anti-aging, functional, and regenerative medicine, as well as having accumulated more than 20 years of experience in emergency medicine and pediatrics, and a master's degree in public health. Dr. Akoury has also served fellowships in pediatric hematology/oncology and performed research in leukemia and the effects of smoking. This lifetime of experience, along with a unique sensitivity, genuine compassion, and driving passion to inspire health in everyone, has prepared "Dr. Dolly" to be in this place at this time. About developing her dream, AWAREmed and Wellness U, Dr. Akoury says, "My mission is to ignite the spark of health deep within everyone, and to allow this sparkle of wellness to shine through everyone's eyes, becoming one with the universe, and aligning body, mind, and spirit." Dalal Akoury, MD, is the founder of AWAREmed Health and Wellness Resource Center and the director of the Wellness U program. Dr. Akoury is board certified in anti-aging, functional, and regenerative medicine, as well as having accumulated more than 20 years of experience in emergency medicine and pediatrics, and a master's degree in public health. Dr. Akoury has also served fellowships in pediatric hematology/oncology and performed research in leukemia and the effects of smoking. This lifetime of experience, along with a unique sensitivity, genuine compassion, and driving passion to inspire health in everyone, has prepared "Dr. Dolly" to be in this place at this time. About developing her dream, AWAREmed and Wellness U, Dr. Akoury says, "My mission is to ignite the spark of health deep within everyone, and to allow this sparkle of wellness to shine through everyone's eyes, becoming one with the universe, and aligning body, mind, and spirit."
|
![]()






 Dalal Akoury, MD, is the founder of AWAREmed Health and Wellness Resource Center and the director of the Wellness U program. Dr. Akoury is board certified in anti-aging, functional, and regenerative medicine, as well as having accumulated more than 20 years of experience in emergency medicine and pediatrics, and a master's degree in public health. Dr. Akoury has also served fellowships in pediatric hematology/oncology and performed research in leukemia and the effects of smoking. This lifetime of experience, along with a unique sensitivity, genuine compassion, and driving passion to inspire health in everyone, has prepared "Dr. Dolly" to be in this place at this time. About developing her dream, AWAREmed and Wellness U, Dr. Akoury says, "My mission is to ignite the spark of health deep within everyone, and to allow this sparkle of wellness to shine through everyone's eyes, becoming one with the universe, and aligning body, mind, and spirit."
Dalal Akoury, MD, is the founder of AWAREmed Health and Wellness Resource Center and the director of the Wellness U program. Dr. Akoury is board certified in anti-aging, functional, and regenerative medicine, as well as having accumulated more than 20 years of experience in emergency medicine and pediatrics, and a master's degree in public health. Dr. Akoury has also served fellowships in pediatric hematology/oncology and performed research in leukemia and the effects of smoking. This lifetime of experience, along with a unique sensitivity, genuine compassion, and driving passion to inspire health in everyone, has prepared "Dr. Dolly" to be in this place at this time. About developing her dream, AWAREmed and Wellness U, Dr. Akoury says, "My mission is to ignite the spark of health deep within everyone, and to allow this sparkle of wellness to shine through everyone's eyes, becoming one with the universe, and aligning body, mind, and spirit."