|
Page 1, 2
Endogenous Steroid Hormone Synthesis and Transport
Steroid hormones are synthesized from cholesterol through a cascade of cytochrome P450 enzymes that oxidize, hydroxylate, and rearrange the cholesterol backbone into specific steroids with unique biological activities. Five categories of steroids are created: estrogens, progestogens, androgens, glucocorticoids, and mineralocorticoids. The most potent of the steroid molecules belonging to each of these categories are estradiol, progesterone, testosterone, cortisol, and aldosterone, respectively. Once synthesized in various glands (ovary, testis, and adrenal) and tissues (liver, adipose), these steroids are released into the bloodstream, where they are rendered more soluble by binding nonspecifically to proteins such as albumin, or more specifically and tightly to sex hormone-binding globulin (binds estrogens and androgens) and cortisol-binding globulin (binds progesterone and cortisol). As the steroid bound to its binding protein percolates through the capillary beds of tissues, a small fraction (about 1%–3%) of the steroid is released into the interstitial tissues and then into target tissues/cells (e.g., estradiol enters the epithelium of the breast and uterus). Whether a steroid has an effect will depend on the presence of specific cellular receptors, which are present in some tissue/cells and not others.
Once the steroid has entered a target cell and the steroid-receptor complex has activated specific gene sites, the complex dissociates and the steroid is available to bind another receptor (recycling) or is metabolized to a weaker steroid (e.g., estradiol to estrone) and/or is released from the target cell back into the intracellular matrix, where it makes its way back to the bloodstream to repeat the cycle or begin the process of elimination.
As the steroid reenters the circulation and passes through the liver, most of it is metabolized via phase I and II enzymes and converted to more water-soluble and inert forms, mostly through sulfation, glucuronidation, and methylation pathways. While these inactive metabolites may continue to circulate in the bloodstream, they can no longer enter target tissues and are eliminated by the kidneys into urine or through bile into the gastrointestinal tract.
Problems and Pitfalls of Testing Sex Hormones in Different Body Fluids Following Different Routes of Exogenous Hormone Supplementation
Testing for sex steroid hormones (estradiol, estriol, estrone, progesterone, and testosterone) is now a mainstay in the clinical evaluation of hormonal imbalances and treatment design for hormone therapy. Steroid hormone levels are most commonly measured in serum/plasma and 24-hour urine collections; however, more biologically relevant and convenient body fluid collections are now available, such as dried capillary blood spots (DBS), saliva, and dried urine.
While most imbalances in endogenous hormones (deficiency and excess) can be detected in any of the four main types of body fluids currently used for testing steroid hormones (i.e., serum, saliva, urine, and DBS), some of these fluids are not appropriate for measuring levels of exogenously delivered steroid hormones, especially those delivered by oral or topical route of administration.
In this mini-review, summarized in Figure 1, I will briefly discuss why some body fluids are not appropriate for testing exogenously delivered hormones, with a focus on the types of tests used and the potential problems following oral steroid administration. More detailed information on the problems with testing in different body fluids with topical hormone administration can be found in the January 2014 issue of Townsend Letter and another journal article that I coauthored.1,2 Therein I elaborate why saliva and DBS, but not venipuncture serum/plasma or urine, are the only way to accurately monitor exogenous topical steroid hormone therapies.
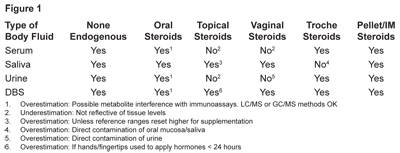
I will also not elaborate extensively on other forms of delivery such as vaginal, transdermal patch, subcutaneous injections, pellet inserts, and sublingual delivery systems, other than to say that we see good correlations in hormone levels following these forms of delivery. The exception to this statement is that the hormone administered cannot be accurately measured in these body fluids if it is applied close to the site of fluid collection (e.g., sublingual/troche hormone use or application of topical hormones to the face or neck and saliva testing; vaginal hormone delivery and urine testing; and/or application of topical hormones with the hands prior to blood collection from the fingertip).
Without going into detail here, our experience with testing and comparing these four body fluids is that all (serum/plasma, DBS, saliva, wet/dry urine) can be reliably used to monitor endogenous levels of the primary active steroid hormones, with the caveat that some tests may lack accuracy if the steroid measured is at very low levels (e.g., blood and salivary estradiol and testosterone), as explained in the next section below.
Pros and Cons of Methods Commonly Used to Measure Steroids in Different Body Fluids
The most common methods of steroid analysis include enzyme immunoassays (EIAs), radio immunoassays (RIAs), and mass spectrometry (LC- and GC-MS/MS). Testing of an individual's endogenous sex hormones in serum has been well characterized in the medical literature, but less so in urine, saliva, and capillary whole blood (DBS).2–10
Enzyme Immunoassays
EIA is the most widely used method today and is incorporated into autoanalyzers used for routine clinical testing of serum and plasma. FDA-approved autoanalyzers are commercially available and have well established and validated methods for testing the sex hormones produced endogenously.3–5 However, testing in autoanalyzers lacks sensitivity for body fluids such as saliva, wherein steroid hormone levels fall below the level of the instrument's sensitivity. More recently, FDA-approved 96-well EIA plates have become available specifically for testing the lower concentrations of steroid hormones in saliva. Despite the FDA stamp of approval, these EIA tests are still a challenge for steroid hormones such as estradiol, which are present in only trace amounts in saliva and subject to matrix effects due to mucins, antibodies, and other nonspecific interfering substances that interact with the antibodies used by the EIA, causing false-high values. This can be circumvented by first extracting the saliva, but most labs test saliva directly without first extracting the steroids to remove these interfering components. All published studies showing good correlation of saliva to serum estradiol have used the extraction method prior to analysis. False-high background levels are most problematic for sex steroids present at very low concentrations, such as estradiol, testosterone, and progesterone, and much less so for DHEA-S and cortisol, which are present at higher concentrations and therefore the matrix effect is only a small fraction of the overall hormone concentration so that it doesn't skew the results.
In conclusion, for accurate, reliable, and reproducible saliva test results for steroids present at trace levels, such as estradiol and testosterone, preextraction prior to testing is essential.
Another consideration with exogenous oral dosing of most sex steroids is that the levels of steroid metabolites can potentially interfere with an immunoassay designed to measure a specific steroid hormone, resulting in false-high values.11 Removing and separating these inert hormone metabolites from the active hormone requires specialized separation techniques such as liquid or solid phase extraction, which is not performed in most commercial diagnostic laboratories prior to serum or saliva testing.3
The degree of cross-reactivity, which causes higher levels of "apparent" hormones but actually represents a combination of active hormone and inactive hormone metabolites, will depend on the quality of the antibody used, which varies depending on the commercial source.3–5
Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS) and Gas Chromatography Tandem Mass Spectrometry (GC/MS/MS)
These methods measure the levels of steroids by a physical method of separation (LC or GC), based on molecular size, followed by mass spectrometry (mass/charge analysis).4,5,12 Because the LC and GC methods separate and differentiate the active steroid from its metabolites, they provide the most accurate and precise assessment of the steroids present in the diagnostic medium. However, such methods require extraction and in some cases derivatization for hormones at low concentration (e.g., estradiol and testosterone).5,12
These methods are also time consuming, require high-level technical input, and are cost prohibitive and difficult to automate, which hinders rapid turnaround time and wide-scale application required by most commercial testing laboratories. Also, until recently, LC and GC mass spectrometry methods were not sensitive enough to detect the very low levels of estradiol and testosterone seen in the saliva and serum of some postmenopausal women and men. Significant improvement in sensitivity of these instruments, along with innovations in methods of derivatization, are rapidly overcoming these hurdles for testing low levels of sex steroids, but such improvements have increased sample processing time and cost, making them still less attractive for broad-scale and cost-effective clinical testing.12
Challenges of Using Different Body Fluids Following Oral Hormone Delivery
Serum Testing and Oral Hormone Delivery
Serum is the most well-characterized body fluid for testing endogenously produced sex steroid hormones, but using it to accurately detect hormones following exogenous oral therapy can be problematic.11 Bioidentical estrogens (estradiol, estriol, estrone), progestogens (progesterone), and androgens (testosterone and DHEA) are all used orally as a form of hormone restoration therapy (HRT) and tested most commonly in serum by FDA-approved automated immunoassays.3,4
What is common to all forms of oral replacement therapies, regardless of the hormone, is that about 10× physiological dosing is required to achieve a physiological level of the active hormone in whatever body fluid is used for testing. Most of the parent hormone administered (e.g., progesterone) is converted to inactive metabolites in the gut. For example, oral estradiol and progesterone dosing are usually in the 0.5 to 1 mg and 100 to 300 mg ranges, respectively, but endogenous peak daily ovarian synthesis of these hormones is 0.05 to 0.1 and 10 to 30 mg.13,14
Some of the commercially available progesterone immunoassays show significant cross-reactivity with progesterone metabolites following oral progesterone therapy, causing false-high progesterone levels in serum. Studies, comparing conventional commercial immunoassays for progesterone with the "gold-standard" and more precise LC/MS, clearly demonstrate that most commercial serum immunoassays that rely on polyclonal antibodies overestimate the true progesterone levels, especially in women using oral progesterone.13,14 Therefore, conventional commercial immunoassays will overestimate serum progesterone following oral therapy, unless more sophisticated methods of extraction are used prior to immunoassay, or the serum is tested by GC or LC mass spectrometry.3–5
Saliva Testing and Oral Hormone Delivery
When the sex hormones are produced endogenously and released into the bloodstream, or they originate from some form of exogenous delivery (most common are oral, topical, vaginal, troche), most of the active hormones (97–98%) are bound up by specific proteins as mentioned earlier. Only a small fraction (about 2%–3%) of the active hormone is released from these binding components in the capillary beds into the interstitial space and tissues.16 Saliva is somewhat unique and different from serum in that most of the polar inert metabolites resulting from oral hormone therapy are filtered out by the salivary gland, only allowing the active hormones to enter saliva.16 Thus, salivary hormones are more representative of not only the amount of the bioactive steroid present in the bloodstream, but also of its bioavailability to target tissues.
Note that while saliva is an excellent diagnostic medium to test hormones delivered orally for the reasons mentioned, timing of collection is important for clinically meaningful results. Orally delivered hormones usually peak in the serum and saliva shortly after supplementation (30 min–2 hours) and then drop precipitously over the next 8 to 12 hours to return to baseline levels.11 Most individuals supplementing with oral progesterone take it twice daily, or only once at night before bed. First morning saliva values are usually at the lower end of the physiological reference range and do not reflect the much higher levels achieved over the first several hours when progesterone would be entering target tissues and binding to and activating cellular receptors. At time points beyond 12 hours, progesterone has usually returned to baseline levels in serum and saliva.15 Therefore, testing salivary progesterone within an 8- to 12-hour time frame after oral dosing, with an established range for this time course, will provide the most clinically meaningful results.
Page 1, 2
|
![]()
![]()
![]()





