Page 1, 2, 3, 4
No adverse effects could be attributed to the diet. At three months, the patient's daily carbohydrate intake was gradually increased to include low glycemic index vegetables while the other restrictions of the diet remained. Laboratory measurements carried out at six weeks and then at 14 months did not show any clinically concerning abnormalities that could be attributed to the diet except for the moderate, but clinically insignificant, increase in the patient's total cholesterol. The patient's low-density lipoprotein cholesterol was moderately elevated at the six-weeks mark but was not remeasured at the 14-month mark, so it is not possible to know if this elevation persisted. At 14 months the patient also had a video EEG that evaluated her sleep, and no evidence of any epileptic activity was found. The patient gained 3 kilograms and grew 6 centimeters while on the diet, and the parents noted improvements in the patient's mood and social functioning. Classic ketogenic diets, on the other hand, can result in notable adverse effects, which were described earlier. Of interest, prior to the diet the patient was considered to be a special needs student, but after being on the diet she was able to perform well enough academically to attend regular school. The only issue that remained, and was not helped by the diet, was that of nocturnal enuresis.
Putative Mechanisms of Action
According to Kossoff et al (2009), the KD interfaces with "a multiplicity of novel molecular targets that respond to a fundamental shift from glycolysis to fatty acid oxidation," but the anti-seizure and possibly the neuroprotective effects of the diet remain unconfirmed (p. 982). Even so, this team of researchers discuss animal data linking the mechanism of the KD to the following: (1) specific ketone bodies (i.e., acetoacetate and acetone) which have anti-seizure effects; (2) decreasing the spontaneous firing rate of GABA (gamma-aminobutyric acid)-ergic neurons in the substantia nigra culminating in membrane hyperpolarization (i.e., lessens neuronal excitation in "other seizure-prone brain areas"); and (3) the abrogation of glycolytic flux (p. 983). Also, the diet does seem to offer some degree of neuroprotection since studies on children have shown that 20% can stop their anti-seizure medications without any recurrence of seizures; and among those that discontinued the diet before reaching one year, 46% had a greater than 50% reduction in seizures for three to six years after (p. 983). Taken together, it is likely that the KD (and any similar variation, such as the ketogenic Paleolithic diet) exerts neuroprotective and/or antiepileptogenic effects.
With respect to the MAD, it is unclear if ketosis is the mechanism behind the diet's efficacy since many children shift out of ketosis over time, yet still have adequate seizure control (Kossoff et al, 2006). It is possible that seizure control results from the initial ketosis induced by the diet, and/or that "low levels of stable ketosis" result in the long-term improvement (Kossoff et al, 2013, p. 440). At this point, it remains unclear how the MAD results in seizure control, but the likely answer involves some combination of increased fat utilization, the elevation of ketones, and other metabolic effects.
Discussion
In my opinion, the MAD is the most promising of all the diets, even though the Paleolithic ketogenic diet was effective in the published case. The MAD diet shows a similar efficacy to that of anti-seizure medication but has fewer adverse effects. Kossoff et al (2013) recommends that the MAD could be offered to new-onset epilepsies for a trial period of one to two months; but if unsuccessful, the diet would then be discontinued and anti-seizure medications would be initiated.
Only one of the patients with CAE from the Johns Hopkins study (Groomes et al, 2011) was on no anti-seizure medication. This patient became seizure-free on the MAD. Thus, these results, except where noted, showed marked clinical benefits when the MAD was added to anti-seizure medication. In the case report, the child with CAE was on no anti-seizure medication and became seizure-free on the Paleolithic ketogenic diet (Clemens et al, 2013).
The fact that diet is not usually advocated as first-line treatment does not mean that it is less effective, and one could argue that it might very well be more effective than anti-seizure medication since it has a much more favourable safety (i.e. adverse effect) profile. The fact that two patients with CAE on no anti-seizure medication became seizure-free suggests more patients with CAE could benefit in a robust manner from diet alone without resorting to anti-seizure medication as first-line treatment. The only issue is that of compliance since the MAD (or Paleolithic ketogenic diet) are much less convenient than anti-seizure medication. Also, it would likely take around one to three months on the MAD, and six weeks on the Paleolithic ketogenic diet (assuming the results of the case report are generalizable) to see discernable clinical benefits, whereas anti-seizure medications typically show benefits earlier even though they do not always put seizures into long-term remission.
Complementary Orthomolecular Treatments - GABA and Phosphatidylserine
GABA
While researching the therapeutic aspects of GABA (i.e., an orthomolecule), I located one of the earliest reports documenting the putative efficacy of GABA among patients with various types of seizures (Tower, 1960). The report contains rich and useful clinical information that was previously unknown to me. Orthomolecules refer to substances found naturally or normally in the human body, such as amino acids, essential fatty acids, hormones, minerals, and vitamins.
The first part of the report presents data on GABA and glutamine metabolism in incubated slices of normal and epileptogenic cerebral cortex (Tower, 1960). In epileptogenic brain slices from humans, the addition of GABA during in vitro incubation completely resolved the abnormality of glutamic acid metabolism (Table 1, p. 563). Based on these results, the conclusion was that "glutamic acid and GABA metabolism may be impaired in most seizure states" (p. 563). These results are consistent with a litany of publications that have implicated abnormal glutamic acid and GABA metabolism in the genesis and chronicity of seizures, including patients with absence seizures (see, for example, Engelborghs et al, 2000).
Another part of the report (Tower, 1960) documented the adverse reactions among three patients that were given GABA only once at a dose of 2 mM/kg (or 0.2 g/kg), and three non-epileptic (i.e., normal) adults that were given a dose of 1 mM/kg (or 0.1 g/kg) only once (p. 566). I have included all the adverse effects that were noted following the oral administration of GABA below:
...within minutesafter ingestion of 2 mM/kg of GABA they experience a sensation of warmth, which coincides with observed flushing of face and extremities. This reaction is often rapidly followed by a general malaise of uneasy feeling, which may be accompanied by nausea and rarely emesis. Two patients have experienced recurrent diarrhea. In addition, the majority of patients experienced paresthesia of the extremities more or less coincident with the foregoing signs and symptoms. Three normal adults on our clinical staff also experienced these reactions after ingestionof a dose of 1 mM/kg. The duration of these various side effects has generally been 10 and 90 min, and tolerance to the compound as far as flushing, malaise and paresthesias are concerned generally develops within a few weeks. In 3 cases the gastrointestinal symptoms have persisted for a number of months, but in no case have been severe enough to necessitate abandoning the therapeutic trial (pp. 566 & 568).
Table 1 lists the possible adverse effects from the oral administration of GABA, including transient throat irritation or self-limited breathing problems that several patients have reported to me; none have been life threatening requiring emergent medical management.
Table 1. Possible Systemic Adverse Effects Following the Oral Administration of a Very High Dose of GABA |
- Flushing
- Paresthesias
- Malaise
- Nausea, vomiting
- Diarrhea
- Transient throat irritation or self-limited breathing problems
|
When explaining the mechanism responsible for these observed adverse effects, Tower (1960) believed they were "peripherally rather than centrally mediated and that they may well be autonomic, perhaps vasomotor phenomena" (p. 568). Moreover, in commenting further about GABA's adverse effects, he reiterated that none were severe or serious and mentioned that "some of our patients have been on daily doses of 8 mM/kg for a year or more with no indication of chronic or cumulative toxicity" (p. 568).
Then the report discussed results from the chronic oral administration of GABA among several patients under the subheading, "Anticonvulsant Effects of GABA in Man" (Tower, 1960, p. 568). The complete report involved a total of 11 patients that were given a regular daily schedule of oral GABA and were followed for three months, and some for as long as two years. The first case involved a 14-year-old girl with petit mal-type seizures. For seven months the patient had been treated with adequate doses of anti-seizure medications (i.e., trimethadione and diphenylhydantoin), but still experienced 300-500 seizures per month (mean: 402±68 seizures per month). When the patient was given oral GABA only (2 mM/kg 4 times daily or 0.8 g/kg daily), there was a "prompt and dramatic reduction of seizure frequency essentially to zero within 2 months" (p. 568). The patient remained essentially seizure-free for six months, and then when GABA was discontinued there was a prompt resumption of seizure activity (at least 5-10 daily; 200-300 seizures per month) during the approximate eight months without it (Figure 1). GABA was resumed slowly (0.5 mM/kg 4 times daily), and then increased to 2 mM/kg four times daily, at which point "a very prompt and undoubted decrease in frequency took place" (p. 569).
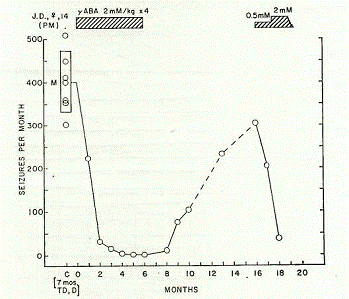
Figure 1. Monthly seizure record of J.D., 14-year-old female. Petit mal (PM) seizure
frequency for the 7-month control period (C) while on trimethadione (TD) and
diphenylhydantoin (D) had a mean value (M) of 402 (±68). Standard deviation
of mean value indicated by length of the box (Tower, 1960, p. 569). Reprinted with
permission from: Tower, D. B. (1960). The administration of gamma-aminobutyric acid to man:
Systemic effects and anticonvulsant action. In Inhibition in the nervous system and
gamma-aminobutyric acid (pp. 562-578). New York, NY: Pergamon Press, Inc.
The EEG prior to the re-administration of GABA revealed "virtually continuous paroxysmal high voltage epileptiform activity induced after 2 min of hyperventilation and persisting to the end of the full 5 min test period" (p. 569). At the end of the 18-month mark (after having resumed GABA for two months) - with an EEG recording made under similar testing circumstances (i.e., to control for any confounds) - the results showed that "during hyperventilation no paroxysmal high voltage activity was observed, and only three brief (2-4 sec) bursts appeared during the whole 5 min hyperventilation test period" (p. 569). Thus, the paroxysmal high-voltage epileptiform activity in the patient's EEG completely disappeared after the readministration of oral GABA during the second trial, with the frequency of seizures having reduced to less than 40 per month (Figure 2).
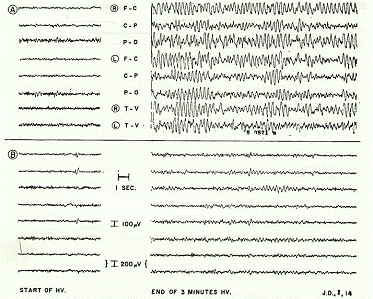
Figure 2. Electroencephalographic records of patient J. D. (Fig. 1) at the start
of hyperventilation (samples on left) and at the end of 3 min of hyperventilation
(sample on the right). Record A taken before second trial of GABA (see Fig. 1)
when frequency of seizures was 200-300 per month. Record B taken at the end of
month 18 (see Fig. 1) while on oral GABA 2mM/kg 4 times daily. Seizure frequency
at this time was less than 40/month. Electrode placements, instrument calibrations and
other conditions were the same for both recordings. Time and calibration scales given
between lower records. Electrode leads given between upper records (abbreviations:
R=right; L=left; F=frontal; C=central; P=parietal; O=occipital – all in the midlateral
plane – T=temporal (ear); and V=mid-vertex) (Tower, 1960, p. 570). Reprinted with
permission from: Tower, D. B. (1960). The administration of gamma-aminobutyric acid to
man: Systemic effects and anticonvulsant action. In Inhibition in the nervous system and gamma-aminobutyric acid (pp. 562-578). New York, NY: Pergamon Press, Inc.
 Other patients were administered GABA to ascertain the effects upon the frequency of seizures. Tower (1960) reported on a 14-year-old boy "with nocturnal generalized (or major) seizures" who also had a clinically significant reduction in seizure frequency when GABA was added to his anti-seizure medications - i.e., mephobarbital and mysoline (pp. 570-571). This patient's seizures always resulted in micturition; but with the reduction in seizure frequency from GABA, his urination issue completely resolved. From looking at this case more closely, it appears that when the patient was treated with the anti-seizure medications listed above and L-asparagine for 15 months, the frequency of his seizures was around 30 per month, but once GABA was added (2 mM/kg 4 times daily) and L-asparagine discontinued, the frequency reduced to around 18 per month during eight months of observation (Figure 3). Other patients were administered GABA to ascertain the effects upon the frequency of seizures. Tower (1960) reported on a 14-year-old boy "with nocturnal generalized (or major) seizures" who also had a clinically significant reduction in seizure frequency when GABA was added to his anti-seizure medications - i.e., mephobarbital and mysoline (pp. 570-571). This patient's seizures always resulted in micturition; but with the reduction in seizure frequency from GABA, his urination issue completely resolved. From looking at this case more closely, it appears that when the patient was treated with the anti-seizure medications listed above and L-asparagine for 15 months, the frequency of his seizures was around 30 per month, but once GABA was added (2 mM/kg 4 times daily) and L-asparagine discontinued, the frequency reduced to around 18 per month during eight months of observation (Figure 3).
A third case (Tower, 1960) involved a 14-year-old girl with diagnoses of minor petit mal-type and major or generalized seizures. During five months on L-asparagine, she was having around 280 minor or petit mal seizures each month and around 150 generalized or major seizures each month. GABA was added (2 mM/kg 4 times daily) and she was followed for 10 months. During this observation period she was on no anti-seizure medication, except for GABA, and the typical premenstrual spike in seizures diminished, the minor or petit mal seizures decreased to approximately 110 per month, and the generalized or major seizures to approximately 115 per month (Figure 3).
Page 1, 2, 3, 4
|
![]()
![]()
![]()
![]()







