Online
publication only
General Information and Statistics
According to the most recent statistics provided by the National Cancer
Institute, breast cancer is (aside from non-melanoma skin cancer)
the number one cause of cancer-related deaths in Hispanic women.
It is the second most common cause of cancer death in white, black,
Asian/Pacific Islander, and American Indian/Alaska Native women.
In 2003 (the most recent numbers available), statistics revealed the following:
· 181,646
women and 1,826 men were diagnosed with breast cancer.*
· 41,619 women and 379 men died from breast cancer.*
In comparison, Figure
1 shows how breast cancer compares to other common causes of death in American
women of all ages.
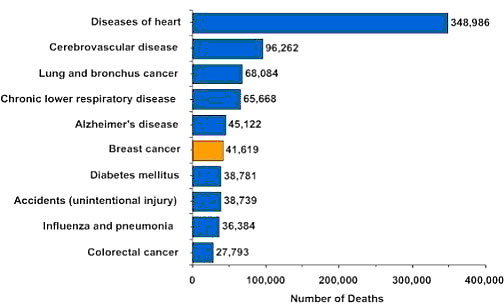
Figure 1: Causes of Death in American Women (2003)
*Note: Incidence counts cover
approximately 96% of the U.S. population and death counts cover 100%
of the US population. Use caution in comparing
incidence and death counts.
Several risk factors have been found that
may increase a woman's
chances of developing breast cancer. These include the following:
· Getting
older
· Early onset of menstrual period
· Starting menopause at a later age
· Being older at the birth of the first child
· Never giving birth
· Not breastfeeding
· Personal history of breast cancer or some non-cancerous breast diseases
· Family history of breast cancer (mother, sister, daughter)
· Treatment with radiation therapy to the breast/chest
· Being overweight (increases risk for breast cancer after menopause)
· Long-term use of hormone replacement therapy (estrogen and progesterone
combined)
· Having changes in the breast cancer-related genes BRCA1 or BRCA2
· Using birth control pills, also called oral contraceptives
· Drinking alcohol (more than one drink a day)
· Not getting regular exercise
Metals and Cancer
Recent research indicated toxic metals, particularly cadmium, nickel,
and aluminum as another cause of breast cancer. These heavy metals
have been known to have
specific effects on several biological systems. Dr. Maggie Louie, assistant
professor in the Department of Natural Sciences and Mathematics, Dominican
University of California, has received a $150,000 grant from the National
Institutes of Health (NIH) in support of breast cancer research
at the University. Dr.
Louie's work focuses on the potential role that environmental contaminants
play in the development of breast cancer.
Dr. Louie is studying how the heavy metal cadmium – an environmental
contaminant that enters the body through consumption of contaminated food or
water, or inhalation of cigarette smoke – contributes to the development
of breast cancer. Her preliminary findings not only show that cadmium promotes
breast cancer cell growth, but her lab may have also identified a potential
pathway for its action.
Breast cancer results from the abnormal growth of cells in the mammary gland.
The development of the mammary gland is regulated by estrogen, a hormone
that binds to the estrogen receptor (ER). Most breast cancer cases initially
develop
as hormone-dependent cancer, in which growth and progression of the disease
correlates with estrogen levels. Doctors have been battling this cancer with
drugs known as anti-estrogens, which are designed to block the receptor.
Although such treatments have proven successful, the cancer can later develop
into a
more aggressive, hormone-independent tumor.
"The mechanism of how hormone independence develops is not clear, and my
current research is focused on understanding the mechanism of how hormone-refractory
breast cancer develops," says Louie. "One potential mechanism may
involve endocrine disruptors including heavy metals such as cadmium." Several
studies conducted by researchers elsewhere back up the theory that cadmium
may enhance the ER function and promote the development of breast cancer.
Louie's
preliminary findings show, however, that cadmium may also activate another
signaling mechanism and promote breast cancer.
Stoica and others documented that cadmium mimics the effects of estradiol
in estrogen-responsive breast cancer cell lines. In addition, cadmium also
blocks
the binding of estradiol to ER-alpha in a noncompetitive manner. This suggests
that cadmium may interact with the hormone-binding domain of the receptor.
Treatment with cadmium resulted in a decrease in estrogen receptor, increased
growth of MCF-7 cell lines, and increased levels of progesterone receptor,
pS2, and cathepsin D. "Results from this study will not only provide
a better understanding of how environmental contaminants such as cadmium
can promote breast cancer, but also offer new insights to how the estrogen
receptor
can regulate both classical and non-classical ER target gene expression," says
Louie.
Research Objective
High levels of transition metals
like iron, nickel, chromium, copper, and lead are closely related
to free radical generation, lipid peroxidation,
formation
of DNA-strand breaks, and tumor growth in cellular systems. Reports in the
last two decades closely relate the presence of transition metals like iron
(Fe) or copper (Cu) to free radical generation via Fenton- and Haber-Weiss-reactions,
ascorbate autoxidation, lipid peroxidation processes, and formation of DNA
strand breaks.2,12,14,19 In turn, lipid peroxidation-induced malondialdehyde-DNA
adducts can accumulate and reach high levels in the breast tissue of women
with breast cancer leading to endogenous DNA modifications.24 Furthermore,
ferric-ethylendiamine N,N'-diacetate (EDDA), and nitrilotriacetic acid
(NTA) complexes were shown to induce free radicals and renal carcinomas in
Wistar rats, demonstrating the key role of transition metals in the abnormal
proliferation process.9,16 As repeated mitochondrial and nuclear DNA mutations
may lead to malignant growth, we (Prof. John G. Ionescu, PhD, Jan Novotny,
MD, Assoc. Prof. Vera Stejskal, PhD, Anette Lätsch, PhD, Eleonore Blaurock-Busch,
PhD, Marita Eisenmann-Klein, MD) investigated the accumulation of 12 heavy
metals in eight healthy and 20 breast cancer biopsies.
Material and Methods
Heavy metal analyses were performed on 20 frozen breast cancer biopsies
and eight healthy breast tissue samples supplied by the Institute
of Pathophysiology
and Oncology, Charles University, Prague, Czech Republic, and the Caritas
Hospital St. Josef, Regensburg, Germany.
The concentrations of iron (Fe), cadmium (Cd), lead (Pb), chromium (Cr),
tin (Sn), nickel (Ni), copper (Cu), mercury (Hg), silver (Ag), gold (Au),
palladium
(Pd), and zinc (Zn) in the biopsy material were measured in the Spezialklinik
Neukirchen,
Germany, by a standardized furnace-atomic absorption spectrohotometry (AAS)-technique
using a Perkin Elmer Sima 6000 AA-spectrophotometer and acidic hydrolysis
as pulping procedure for sample preparation.
Additionally, heavy metal analysis in all control biopsies was done by using
an inductive coupled plasma-mass spectroscopy (ICP-MS) with cell technique
in the Laboratory for Micro Trace Minerals, Hersbruck, Germany. All tests
were performed three times.
The result per sample is the mean value of three determinations expressed
in µg/kg. Table 1
Heavy metal content in breast cancer (n = 20) and healthy
breast tissue (n = 8) biopsies (12KB .pdf)
Results
The Mann-Whitney U Test was used for
statistical analysis of the results. A highly significant accumulation
of iron (p < 0.0001), nickel (p < 0.00005),
chromium (p < 0.00005), zinc (p < 0.00001), cadmium (p < 0.005),
mercury (p < 0.005), and lead (p < 0.05) was recorded in the cancer
samples when compared to the control group. Copper and silver showed no significant
differences to the control group, whereas tin, gold, and palladium were not
detectable in any biopsies. (See Table 1.) There was no statistical difference
in the heavy metal content of the control biopsies when analyzed by AAS or
ICP-MS (data not shown).
Discussion
In biological systems, the concentration of redox-active transition metals
capable of catalyzing and/or generating free radicals like superoxide, hydrogen
peroxide, and hydroxyl radical appears to be relatively low. However, under
certain pathological conditions (haemochromatosis, Wilson disease, collagenoses,
and various malignancies), transition metals and their transport proteins
may accumulate in different target organs inducing cellular lipid peroxidation
and DNA-attack.
In this respect, the ability of excess Fe in mediating the formation of hydroxyl
radicals, suppressing cellular immune functions, and promoting tumor growth
is well-established. Increased Cu concentrations were also found in human lung
cancer biopsies and in other tumors. Ni, Cr, and Cd have been recognized as
mutagens and carcinogens through their ability to inhibit the repair of damaged
DNA. In addition, they can enhance the mutagenicity and carcinogenicity of
directly-acting genotoxic agents. At the same time, carcinogenic effects of
Ni, directly or in association with organic compounds, have been described
in the literature, and recently, higher concentrations of Fe and Ni have been
found in the malignant human prostate. Inhaled particulate forms of hexavalent
Cr cause lung cancer, and at the cellular level, Cr exposure may lead to cell
cycle arrest, apoptosis, or neoplastic transformation.
Occupational exposure to Cd is associated with lung cancer in humans, and high
Cd concentrations have been found in proliferative prostate lesions. Interestingly,
Zn, as an essential element, was shown to mediate and increase tumor growth,
and Zn depletion was shown to suppress tumor growth in mice and rats. Macromolecular
compounds (dextrans) substituted with Hg-containing side chains were reported
to promote fibrosarcoma growth in mice.
The etiology of the majority of human breast cancers is still controversial.
However, hormonal influences and environmental toxic compounds inducing oxidative
stress and lipid peroxidation have been suggested to play a role in breast
carcinogenesis. Our data describe for the first time a major accumulation of
Fe and other transition metals like Ni, Cr, Cd, Zn, Hg, and Pb in the breast
cancer tissue with implications in the pathogenesis of breast cancer.
Conclusions
These data suggest that unphysiological gradual accumulation of transition
metals in the breast tissue may be closely related to the malignant growth
process. Evaluation of metal exposure through blood, hair, or urine provocation
tests, and subsequent chelation treatment may be needed to prevent and treat
metal-related breast cancer and other malignancies.

Authors
Blaurock-Busch Eleonore, PhD, Research Department, Laboratory for Micro
Trace
Minerals, Hersbruck, Germany
Eisenmann-Klein Marita, MD, Caritas Hospital St. Josef, Regensburg, Germany
Ionescu Prof. John G., PhD, Research Department of Spezialklinik Neukirchen,
Neukirchen, Germany
Lätsch Anette, PhD, Research Department of Spezialklinik Neukirchen, Neukirchen,
Germany
Novotny Jan, MD, Institute of Pathophysiology and Oncology, Charles University,
Prague, Czech Republic
Stejskal Assoc. Prof. Vera, PhD, Department of Clinical Chemistry, Danderyd Hospital
and Karolinska Institute, Stockholm, Sweden 
A list of additional references is available upon request.
Editor's note: only a few of the citations
listed are noted in the text.
Notes
1. Adachi S, Takemoto K, Ohshima S, Shimizu Y, Takahama M. Metal concentrations
in lung tissue of subjects suffering from lung cancer. Int
Arch Occup Environ Health. 1991; 63:193-197.
2. Aust SD, Morehouse LA, Thomas CE. Role of metals in oxygen radical
reactions. J Free Radic Biol Med. 1985; 1: 3-25.
3. Baader SL, Bruchelt G, Carmine TC, Lode HN, Rieth AG, Niethammer
D. Ascorbic-acid-mediated iron release from cellular ferritin and its
relation to the formation of DNA strand breaks in neuroblastoma cells. J
Cancer Res Clin Oncol. 1994; 120 (7):415-421.
4. Beyersmann D. Effects of carcinogenic metals on gene expression.
Toxicol Lett. 2002; 127(1-3), 63-68.
5. Institute of Medicine. National Research Council. Lifestyle Behaviors
Contributing to the Burden of Cancer. In: Curry S, Byers T, & Hewitt
M, editors. Fulfilling the Potential of Cancer
Prevention and Early Detection. Washington, DC: The National Academies Press; 2003:41-86.
6. Ebadi M, Swanson S. The status of zinc, copper and methallothionein
in cancer patients. Prog Clin Biol Res. 1988; 259:161-175.
7. Hartwing A. Recent advances in metal carcinogenicity. Pure
ApplChem.
2000; 72: 10071014.
8. Ionescu JG. New evidence-based therapies for cancer. Proceedings
of the 17th Int. Symposium on Integrative Medicine, p.1-21, Tenerife,
Spain, June 2005.
9. Ionescu JG. Transition metals and cancer. Communication at the 12th
MELISA Study Group Conference, Prague, September 2005.
10. Liu M, Okada S. Induction of free radicals and tumors in the kidney
of Wistar rats by ferric ethylendiaminbe-N,N'-diacetate. Int
J Sports Med. 1996; 17:397-403.
11. Lode HN, Bruchelt G, Zinsser D, Baader SL, Rieth AG, Schade UF,
et al. Ascorbic acid induces lipid peroxidation on neuroectodermal
SK-N-LO cells with high endogenous ferritin content and loaded with
Mab-ferritin immunoconjugates. Anticancer
Res. 1994; 14 (5A):1903-1906.
12. McQuitty JT Jr, DeWys WD, Monaco L, Strain WH, Tob CG, Apgar J,
et al. Inhibition of tumor growth by dietary zinc deficiency. Cancer
Res. 1970; 30(5):1387-1390.
13. Mello FA, Meneghini R. In vivo formation of single-strand breaks
in DNA by hydrogen peroxide is mediated by the Haber-Weiss-reaction.
Biochem Biophys Acta. 1984; 781: 56 63.
14. Mills BJ, Broghamer WL, Higgins PJ, Lindeman RD. Inhibition of
tumor growth by zinc depletion of rats. J
Nutr. 1984; 114 (4):746-752.
15. Minotti G, Aust SD. The requirements for iron (III) in the initiation
of lipid peroxidation by iron (II) and hydrogen peroxide. J
Biol Chem.
1987; 262:1098-1104.
16. Ohmori T, Okada K, Tabei R, Shibata T. Effects on tumor induction,
growth, metastasis and histology of concurrent administration of putrescine
and its metabolising inhibitor alphadefluoromethylornithine in nickel
tumorigenesis in soft tissue. Carcinogenesis. 1994; 15 (4):647-652.
17. Okada S. Iron-induced tissue damage and cancer: the role of reactive
oxygen species and free radicals. Pathol Int. 1996; 46:311-332.
18. Pierini G, Fini M, Giaveresi G, Dallari S, Brayda BM, Rocca M,
et al. Atomic absorption spectrophotometry (AAS) for the evaluation
of metallosis in prostheses and artificial organs: a new approach.
Int J Artif Organs. 1999: 22 (7):522-527..
19. Pitha J, Kociolek K, Apffel CA. Opposite effects of dextrans substituted
with sulfhydryls or mercury on tumor growth. Cancer
Res. 1979; 39 (1):170-173.
20. Scarpa M, Stevanato R, Viglino P, Rigo A. Superoxide ion as active
intermediate in the autoxidation of ascorbate by molecular oxygen.
J Biol Chem. 1983; 258:6695-6697.
21. Singh J., Carlisle DL, Pritchard DE, Patierno SR. Chromium-induced
genotoxicity and apoptosis: relationship to chromium carcinogenesis
(review). Oncol Rep. 1998; 5 (6):1307-1318.
22. Stejskal VD, Danersund A, Lindvall A, Hudecek R, Nordman V, Yaqob
A, et al. Metal-specific lymphocytes: biomarkers of sensitivity in
man. Neuroendocrinol Lett. 1999; 20:289-298.
23. Stewart BW & Kleihues P, editors. World
Cancer Report. France:
IARC Press: 2003.
24. Takeda A, Goto K, Okada S. Zinc depletion suppresses tumor growth
in mice. Biol Trace Elem Res. 1997; 59(1-3):23-29.
25. U.S. Cancer Statistics Working Group. United
States Cancer Statistics: 2003 Incidence and Mortality. Atlanta (GA): Department of Health and
Human Services, Centers for Disease Control and Prevention, and National
Cancer Institute; 2007, and National Vital
Statistics Vol. 53, No.
5, 2004.
26. Vainio H & Bianchini F, editors. International Agency for Research
on Cancer. Evaluation. In: IARC Handbooks
of Cancer Prevention: Weight Control & Physical Activity. France: IARC Press: 2002. p. 249–250.
27. Waalkes MP, Coogan TP, Carter RA. Toxicological principles of metal
carcinogenesis with special emphasis on cadmium. Crit
Rev Toxicol.
1992; 22:175-201.
28. Wang M, Dhingra K, Hittelman WN, Liehr JG, de Andrade M, Li D.
Lipid peroxidation-induced putative malondialdehye-DNA adducts in human
breast tissue. Cancer Epidemiol Biomarkers
Prev. 1996; 5:705-710.
29. Weinberg ED: The role of iron in cancer. Eur
J Cancer Prev. 1996;
5:19-36.
30. Angelo Wong, Rachel Puckett, Jessica Assaf, and (Dr. Maggie C.
Louie) Department of Natural Sciences and Mathematics, Dominican University
of California, San Rafael, CA 94901 Effects of Heavy Metals on Breast
Cancer Proliferation
31. Yaman M, Atici D, Bakirdere S, Akdeniz I. Comparison of trace metal
concentrations in malign and benign human prostate. J
Med Chem. 2005;
48:630-634.
32. National Cancer Institute. Breast Cancer
PDQ: Prevention - Health
Professional , Breast Cancer PDQ:
Prevention - Patient , Breast
Cancer PDQ: Treatment - Health Professional., National
Cancer Institute. Breast Cancer PDQ: Treatment
- Patient.
|



![]()
![]()


