|
![]()
From the Townsend Letter |
||||
The Role of Regenerative Medicine in Breast Reconstruction by Karina Gordin, MSc |
||||
Page 1, 2 Breast augmentation is among the most common cosmetic surgical procedure in the US, with approximately 106,000 reconstructive plastic surgeries performed in breast cancer patients post-mastectomy.1 Silicone and saline implants are routine options, despite mixed outcomes and reports of complications, most notably capsular contracture, numbness, leaking and breaking, as well as reoperations involving implant replacement and removal.2 Regarding the latter, approximately 42,000 implant removals were performed in 2015, 17,892 of which comprised reconstructive cases.3 Considering these statistics, it is unsurprising that in spite of the increased number of breast augmentations, debates persist about the safety of implants. Trusted health advocate, renowned actress, and New York Times bestselling author Suzanne Somers has been at the forefront of the breast reconstruction debate, advocating for alternative treatment options that harness the body's own capacity to heal and recover. One such option is cell-assisted lipotransfer (CAL) – a groundbreaking regenerative medical procedure that regrows the breast, rather than reconstructs it. Namely, CAL is based on the premise of autologous fat grafting, which utilize the patient's own subcutaneous fat tissue (from regions like abdomen and lower legs) as implant for the breast. However, unlike conventional fat grafting, which demonstrates relatively poor long-term viability,4 cell-assisted lipotransfer enhances fat graft survival rate via autologous adipose-derived stem cells (ADSC). In fact, studies5 have demonstrated that ADSC can survive the period of hypoxia post-surgery, which is thought to result in the necrosis of conventional fat. Adipose-derived stem cells enhance both angiogenesis and adipogenesis,6 which translates well in the clinical setting, with long-term graft retention and lower post-operative complications.7,8 Karina Gordin (KG): You spearheaded the first US-based clinical trial examining cell-assisted lipotransfer (CAL) in breast reconstruction. Can you please set the scene for your interest in regenerative medicine, and the path that led to your becoming the first patient in the United States to undergo the experimental procedure? Suzanne Somers (SS): I had read about a doctor at the University of Tokyo- Dr. Kotaro Yoshimura- a wonderful man who had successfully regrown the breasts of over 400 Japanese women using stem cells and fat transfer. I took a chance and called him and to my great surprise and good luck, he knew who I was. We talked often on the phone and eventually he agreed to come to Los Angeles and meet with Dr. Joel Aronowitz at Cedars-Sinai Plastic and Reconstructive Surgery Center in Los Angeles. Dr. Aronowitz was very interested in working with stem cell therapy, and Dr. Yoshimura taught him the procedure. But it wasn't that simple. I needed to apply for an Institutional Review Board to qualify me for a clinical trial; that took three years! The day it was granted, July 2011, we went to work to begin the procedure, and my surgery was performed in August.
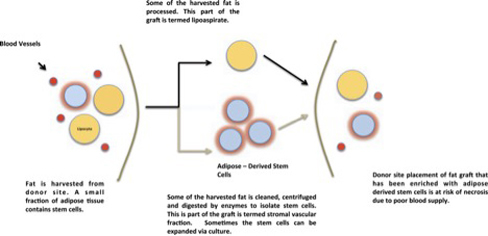
SS: When I had a lumpectomy to remove a 2.4 cm tumor, I lost half my right breast. I had no chemo, but the 35 days of radiation treatments destroyed tissues in my right breast, leaving it small and flat, but with the nipple intact. During the 11 years missing half of the breast was something I just accepted. It didn't affect me negatively but reminded me how lucky I was to have found cancer early enough, and that I had the courage to walk away from conventional medicine. It wasn't a reckless decision. I had researched it, and the idea of pumping my body with chemical poison just did not sit well. Instead I chose to change my diet and lifestyle, and I also injected Iscador, a mistletoe extract that I was able to get from Europe which builds the immune system. I am forever grateful I made these decisions for myself. KG: How long did the procedure take? SS: Only a couple of hours for the entire procedure of removing some of my fat through liposuction, whipping it in a mechanism akin to a centrifuge to separate it into three layers: fat, blood, and stem cells. The stem cells were harvested and filtered; weak stem cells were discarded. Then my stem cell-enriched autologous fat was injected under and over the tissue of my right breast, lifting the breast to match the dimension and position of my left breast in a process that took 10 to 15 minutes. Figure 1: Cell-Assisted Lipotransfer Procedure
KG: Regarding long-term effects, clinical data about the inherent safety and viability of CAL are limited. Accordingly, questions con-cerning risks like neoplasia and calcification remain. Can you please describe your experience with CAL in terms of safety, side effects, pain and recovery? And have you received follow-up procedures? Page 1, 2
|
||||
![]()
Consult your doctor before using any of the treatments found within this site.
![]()
Subscriptions
are available for
Townsend Letter, the Examiner of Alternative Medicine
magazine, which is
published 10 times each year. Search our pre-2001
archives for further information. Older issues of the printed magazine
are also indexed for your convenience.
1983-2001
indices ; recent indices. Once you find the magazines you'd like to order, please
use our
convenient form, e-mail subscriptions@townsendletter.com,
or call 360.385.6021.
360.385.6021
Fax: 360.385.0699
info@townsendletter.com
Who are
we? | New articles | Featured
topics | e-Edition |
Tables of contents | Subscriptions | Contact
us | Links | Classifieds | Advertise |
Alternative
Medicine Conference Calendar | Search site | Archives |
EDTA Chelation Therapy | Home
© 1983-2017 Townsend Letter
All rights reserved.
Website by Sandy
Hershelman Designs
![]()