Abstract
Patients with neurodegenerative diseases and behavioral disorders
often have systemic bacterial, viral, and/or fungal infections that
are important in disease progression and severity. We and others
have examined patients with various neurodegenerative and behavioral
neurological conditions, such as Amyotrophic Lateral Sclerosis (ALS),
Multiple Sclerosis (MS), Alzheimer's disease (AD) and Autistic
Spectrum Disorders ([ASD], including Autism, Attention Deficit Disorder,
Asperger Syndrome), and found evidence for systemic intracellular
bacterial and viral infections in a majority of patients. For example,
examination of blood for evidence of Mycoplasma species, Chlamydia
pneumoniae, Brucella species, Borrelia burgdorferi, and other infections
by serology, Western blot, or polymerase chain reaction revealed
high incidences of systemic co-infections that were not found in
control subjects (P<0.001). The results were compared to other
chronic illnesses where neurological manifestations are often found,
such as Chronic Fatigue Syndrome/Myalgic Encephlomyopathy (CFS/ME),
Fibromyalgia Syndrome (FMS), Lyme disease (LD), and Gulf War illnesses.
Most of these chronic illness patients also had multiple intracellular
bacterial infections compared to control subjects (P<0.001),
and the most common co-infection found was Mycoplasma species in
all of the conditions examined. In contrast, in the few control
subjects that tested positive, only single infections were found.
The results suggest multiple chronic intracellular bacterial (and
viral) infections are common features of neurodegenerative and behavioral
disorders, and treatment regimens should address the multiple infections
present in these conditions.
Introduction
Neurodegenerative diseases are chronic degenerative diseases of
the central nervous system (CNS) that often cause dementia in the
aging population. For the most part, the causes and mechanisms of
this collection of brain diseases remain largely unknown, and they
are increasing in incidence in the developed as well as the underdeveloped
world.1 These diseases are characterized by molecular changes in
nerve cells that result in nerve cell degeneration and, ultimately,
nerve dysfunction and cell death, resulting in neurological signs
and symptoms and, eventually, dementia.1,2
There appears to be a genetic link to neurodegenerative diseases,
but the genetic changes that occur and the changes in gene expression
that are found in these diseases are complex and not related to
simple genetic mutations, deletions, or amplifications.1 In addition
to genetic changes and changes in gene expression, it is thought
that nutritional deficiencies, head trauma, environmental toxins,
chronic bacterial and viral infections, autoimmune immunological
responses, vascular diseases, accumulation of fluid in the brain,
changes in neurotransmitter concentrations, and other causes are
involved in various neurodegenerative diseases.1-5 One of the biochemical
changes found in essentially all neurological degenerative diseases
is the over-expression of oxidative free radical compounds (oxidative
stress) that cause lipid, protein, and genetic structural changes.3,4
An attractive model for neurodegeneration resulting in neurological
disease involves the action of toxic products produced as a result
of chronic bacterial and/or viral infections.6,7 Infectious agents
may enter the CNS within infected migratory macrophages, or they
may gain access by transcytosis across the blood-brain-barrier or
by intraneuronal transfer from peripheral nerves.6 Cell wall-deficient
bacteria, principally species of Mycoplasma, Chlamydia, Coxiella,
Brucella, Borrelia, among others, are candidate infectious agents
that may play an important role in neurodegenerative diseases.8
Such infections may also cause disease progression, and since they
are usually systemic, they could affect the immune system and other
organ systems, resulting in systemic signs and symptoms.9
Methods
Blood Collection
Blood was collected, immediately
brought to ice bath temperature and shipped with wet ice by air
courier to the Institute for Molecular Medicine for analysis. All
blood samples were blinded. Whole blood was used for preparation
of sera or DNA using Chelex as previously described.10,11 Multiple
tests were performed on all patients and control subjects.
Western Blot of
Borrelia burgdorferi
Patients were recruited who were previously tested for Borrelia
burgdorferi using Western Blot analysis.12,13 Laboratory results
were examined, and criteria for a positive Western blot was that
at least two of the Borrelia burgdorferi genus-specific antigens
(18K, 23K, 30K, 31K, 34K, 37K, 39K, 83K, and 93K) were reactive
in Western blots.
Amplification
of Gene Sequences by PCR
Amplification of the target gene sequences by Polymerase Chain Reaction
(PCR) was accomplished as previously described.10,11 Negative and
positive controls were present in each experimental run. The amplified
samples were separated by agarose gel electrophoresis. After denaturing
and neutralization, Southern blotting was performed to confirm the
PCR product.10,11 Multiple PCR primer sets were used for each species
tested to minimize the chance that cross-reacting microorganisms
were detected.
Statistics
Subjects' demographic characteristics were assessed using
descriptive statistics and students' t-tests (independent
samples test, t-test for equality of means, 2-tailed). Pearson Chi-Square
test was performed to compare prevalence data between patients and
control subjects.
Amyotrophic Lateral
Sclerosis
Amyotrophic Lateral Sclerosis (ALS) is an adult-onset, idiopathic,
progressive degenerative disease affecting both central and peripheral
motor neurons. Patients with ALS show gradual progressive weakness
and paralysis of muscles due to destruction of upper motor neurons
in the motor cortex and lower motor neurons in the brain stem and
spinal cord, ultimately resulting in death, usually by respiratory
failure.14,15 The overall clinical picture of ALS can
vary, depending on the location and progression of pathological
changes found in nervous tissue.16
In ALS patients, the role of chronic infections has attracted attention
with the finding of enterovirus sequences in a majority of spinal
cord samples by PCR.17,18 Although others have failed
to detect enterovirus sequences in spinal cord samples from patients
with or without ALS,19 infectious agent(s) may play a
role in the etiology of ALS. We studied the presence of systemic
microbial infections in a preliminary number of ALS patients.20
We found that 8/8 Gulf War veterans (from three nations) diagnosed
with ALS had systemic Mycoplasmal infections. All but one patient
had M. fermentans infections, and one veteran from Australia had
a systemic M. genitalium infection. In 22/28 nonmilitary ALS patients
from the US, Canada, and Great Britain, we also found blood Mycoplasmal
infections. Of the Mycoplasma-positive civilian patients who were
further tested for M. penetrans, M. fermentans, M. hominis, and
M. pneumoniae, most were positive for M. fermentans (13/22, 59%),
but we did find other Mycoplasma species, such as M. hominis (7/22,
31%) and M. pneumoniae infections (2/22, 9%). Two civilian ALS patients
had multiple Mycoplasmal infections (M. fermentans plus M. hominis,
9%). Multiple Mycoplasmal infections were not found in the military
patients with ALS. The difference in incidence of Mycoplasmal infections
between ALS patients and control subjects was highly significant
(P<0.001).20 The incidence of various chronic bacterial
and viral infections in ALS is shown in Figure
1. The other type of infection that is commonly found in
ALS is Lyme Borrelia burgdorferi (Figure 1).
Thus, a byproduct of Lyme disease may be progression to ALS, but
this is probably only possible in some Lyme disease patients who
have the genetic susceptibility genes for the neurodegenerative
disease.21
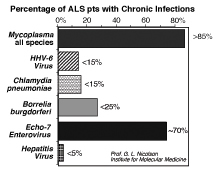 Figure
1: Percent incidence of systemic bacterial
and viral infections in 46 patients with Amyotrophic Lateral Sclerosis.
The results were determined by Western blot or PCR. Figure
1: Percent incidence of systemic bacterial
and viral infections in 46 patients with Amyotrophic Lateral Sclerosis.
The results were determined by Western blot or PCR.
ALS patients also have other chronic infections, including Human
Herpes Virus-6 (HHV-6), Chlamydia pneumoniae, and, as mentioned
above, Borrelia burgdorferi but rarely hepatitis virus (Figure
1). Similar to the possible role of enteroviruses in the
pathogenesis of ALS, the exact role that the other infections play
in the pathogenesis or progression of ALS is not known. They could
be cofactors in the pathogenesis of ALS, or they could simply be
opportunistic infections that cause morbidity in ALS patients, such
as the respiratory, rheumatic symptoms, and other problems often
found in ALS patients. They could also be involved in the progression
of ALS rather than in its inception.
Although the exact cause of ALS remains unknown, there are several
hypotheses on its pathogenesis: (a) accumulation of glutamate causing
excitotoxicity; (b) autoimmune reactions against motor neurons;
(c) deficiency of nerve growth factor; (d) dysfunction of superoxide
dismutase due to mutations; and (e) chronic infection(s).16-23
Future studies should determine more precisely the role of chronic
bacterial and viral infections in ALS pathogenesis and progression.
Multiple Sclerosis
Multiple Sclerosis (MS) is a disease of the nerves of the central
nervous system, and it can occur in young as well as older people.
The nerves in various parts of the brain are covered by a protective
insulation containing the protein myelin and other proteins embedded
in a lipid sheath so that the electrical impulses that cause nerve
conduction are protected. In MS, inflammation and the presence of
autoimmune antibodies against myelin and other nerve cell antigens
cause the protective sheath to break down (demyelination), resulting
in decrease or loss of electrical impulses along the nerve.
In progressive MS, the nerve cells are damaged additionally by the
deposition of plaques on the nerve cells to the point where nerve
cell death occurs. There is also breakdown of the blood-brain barrier
associated with local inflammation caused by glial cells.24,25
The clinical results of demyelination and blood-brain barrier lesions
are variable but usually include impaired vision, alterations in
motor, sensory, and coordination systems and cognitive dysfunction.
Often, these are cyclic (relapsing-remitting) over some time, but
a subgroup of patients' progress more rapidly.25
A possible infectious cause for MS has been under investigation
for approximately the last decade.25-27 Epidemiological
and twin studies suggest that MS is acquired not inherited. Since
more than 90% of MS patients show immunological and cytokine characteristic
of infection, MS patients have been examined for various viral and
bacterial infections. One of the most common findings is the presence
of Chlamydia pneumoniae in MS brains,28-30 although this
has not been found in every study.31,32 A possible reason
for this is that other infections could also be involved. In addition
to Chlamydia pneumoniae found in some studies, MS patients could
have Mycoplasma species, Borrelia burgdorferi, Human Herpes Virus-6,
and other infections.
Recent research at the Institute for Molecular Medicine and elsewhere
has shown that autoimmune responses to nerve cell proteins may be
caused, in part, by intracellular bacterial infections. As many
as 80% of MS patients may have intracellular bacterial infections
caused by Mycoplasma, Chlamydia, and other cell wall-deficient bacteria
species that were found only at low incidence in age-matched subjects
(P<0.001). Additional bacterial infections, such as Borrelia
burgdorferi (Lyme disease), and other intracellular bacterial infections
may also be involved in some MS cases (Figure
2). When these infections are released from cells, they contain
host cell antigens in their exterior membranes, and these normal
cell membrane antigens could stimulate autoimmune responses. Alternatively,
the microorganisms may express antigens that mimic normal surface
antigens.
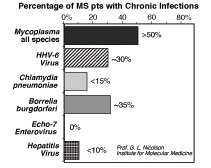 Figure
2: Percent incidence of systemic bacterial
and viral infections in 65 patients with Multiple Sclerosis. The
results were determined by Western blot or PCR. Figure
2: Percent incidence of systemic bacterial
and viral infections in 65 patients with Multiple Sclerosis. The
results were determined by Western blot or PCR.
Viruses may also be involved in MS.33 Certain viruses
have been found at high incidence in MS patients, such as Human
Herpes Virus-6 (HHV-6).33 We have also found this virus
in the systemic circulation of MS patients (Figure
2), suggesting that it might be involved in the pathogenesis
of MS. Viruses may stimulate autoimmune responses when they kill
cells, resulting in release of normal antigens into the surrounding
extracellular environment.
Since infections usually stimulate immunological responses, the
presence of intracellular bacterial infections in nerve cells, in
particular, may stimulate autoimmune responses against nerve cell
antigens. In the case of MS, some 20 different bacterial and viral
infections have been found, but the link between these infections
and the pathogenesis of MS is still being debated.34
Perhaps this is the reason that one or even a few types of infections
cannot be linked to every case of MS. That, however, does not prove
that infections, in general, are not linked to the pathogenesis
of MS.
Does other evidence suggest that infections may be involved in the
pathogenesis of MS and other neurological diseases? The answer to
this question is most certainly, yes. These diseases can progress
to a fatal phase, especially when intracellular infections are found.29
Upon autopsy, intracellular bacteria, such as C. pneumoniae and
Mycoplasma species, have been found at high levels inside nerve
cells in the CNS,34,35 The presence of such bacteria
has been linked to various neurological diseases.29,30
In addition, control infection of non-human primates with cell-invading
bacteria, such as Mycoplasma fermentans, results in a fatal disease
with neurological complications.36 When these infected
brains are examined at autopsy, the Mycoplasma fermentans can be
found in brain tissue.36
Alzheimer's Disease
Alzheimer's disease (AD), the most common cause of dementia, is
a collection of brain disorders usually found in older people. The
disease is characterized by slow, progressive loss of brain function,
especially notable by lapses in memory, disorientation, confusion,
mood swings, changes in personality, language problems, such as
difficulty in finding the right words for everyday objects, loss
of behavioral inhibitions, loss of motivation, and paranoia. The
prognosis and course of AD varies widely, and the duration of illness
can be a few years to over 20 years in duration. During this time,
the parts of the brain that control memory and thinking are the
first affected, followed by other brain changes that ultimately
result in brain cell death.37 AD is characterized by distinct neuropathological
changes in the brain. Among the most notable are the appearance
of plaques and tangles of neurofibrils within brain nerves that
affect nerve synapses and nerve-nerve cell communication. Both of
these structures involve the deposition of altered amyloid proteins,
called Ab proteins.38,39
Although the cause of AD is not known to any certainty, the formation
of the amyloid plaques and neurofiber tangles may be due to genetic
defects and resulting changes in the structure of Ab proteins, neurotoxicity
caused by chemicals or other toxic events, inflammatory responses,
oxidative stress and increases in ROS, loss of nerve trophic factors
that are important in nerve physiology, and loss of nerve cell transmission.38-42
Brain infections in AD have only recently become an important topic.43,44
One pathogen that has attracted considerable attention is Chlamydia
pneumoniae.45,46 This intracellular bacteria has a tropism for neural
tissue,46 and it has been found at high incidence in the brains
of AD patients by PCR and immunohistochemistry methods. C. pneumoniae
bacteria have been found in nerve cells in close proximity to neurofibrillary
tangles.47,48 The infection results in endothelial cell invasion
and promotes the transmigration of monocytes through human brain
endothelial cells into the brain parenchyma.49 Although C. pneumoniae
has been found in the brains of most AD patients studied,42,46 and
this infection results in amyloid beta (Abeta) plaque formation
in mice injected with C. pneumoniae,50 some studies have not found
an association with Alzheimer's using PCR51 or immunohistochemistry.52
In addition to C. pneumoniae, evidence has been forthcoming that
Alzheimer's disease patients also have other infections, such as
Lyme disease Borrelia burgdorferi.53 This infection has been confirmed
in Alzheimer's disease by serology, culture, Western blot, and immunofluorenscence.54-56
In fact, the presence of intracellular infections like Borrelia
burgdorferi found in AD are thought by MacDonald57 to be the primary
event in the formation of AD amyloid plaques by forming "congophilic
cores" that attract amyloid materials. In addition, the induction
of ROS, lipid peroxidation, and the breakdown of the lysosomal membrane,
releasing lysosomal hydrolases, are also thought to be important
in amyloid deposition.58 Most reports show that AD nerve cells are
positive for Borrelia burgdorferi in AD,53-57 but there are also
some negative reports.59 As expected, Borrelia burgdorferi co-infections
are found in AD, and an interesting relationship has developed between
the presence of Borrelia burgdorferi and Herpes Simplex Virus-1
(HSV1) in AD.60 It had been noted previously that HSV1, but not
a related neurotrophic virus (Varicella Zoster Virus), was found
often in AD brains and may be linked to patients who have the AD
risk factor apoE4 allele.61,62 HSV1 is thought to be involved in
the abnormal aggregation of beta amyloid or Abeta within the brain
by reducing the amount of full length amyloid precurser protein
and increasing the amount of the Abeta fragment from this precursor.63
Autistic Spectrum
Disorders
Children with Autistic Spectrum Disorders (ASD), such as autism,
attention deficit disorder, Asperger syndrome, etc., generally suffer
from an inability to properly communicate, form relationships with
others, and respond appropriately to their environment. Such patients
do not all share the same signs and symptoms but tend to share certain
social, communication, motor, and sensory problems that affect their
behavior in predictable ways. These children often display repetitive
actions and develop troublesome fixations with specific objects,
and they are often painfully sensitive to certain sounds, tastes
and smells.64,65 The signs and symptoms of ASD are thought
to be due to abnormalities in brain function or structure. In some
ASD patients, there are also a number of other less specific chronic
signs and symptoms. Among these are fatigue, headaches, gastrointestinal
and vision problems, occasional intermittent low-grade fevers, and
other signs and symptoms that are generally excluded in the diagnosis
of ASD.
The causes of ASD are unknown and may include genetic defects and
heavy metal, chemical, and biological exposures, among others, and
are probably different in each patient.64,65 However,
among ASD patients, there may be similarities in genetic defects
and environmental exposures that are important in patient morbidity
(sickness) or in illness progression. Other chronic illnesses have
some of the same chronic signs and symptoms, suggesting that there
may be some overlap in the underlying causes of these conditions
or at least in the factors that cause illness or morbidity or illness
progression.
Chronic infections appear to be an important element in the development
of ASD.66 Such infections are usually held in check by
immune surveillance, but they can take hold and become a problem
if they can avoid host immunity and penetrate and hide in various
tissues and organs, including cells of the CNS and peripheral nervous
system. When such infections occur, they may cause many of the complex
signs and symptoms seen in various chronic illnesses.66-68
Changes in environmental responses and increased titers to various
endogenous viruses as well as bacterial and fungal infections commonly
have been seen in ASD along with the presence of heavy metals.64,65
In ASD, there is an interesting but widely contested relationship
between the disease, heavy metals, and vaccines. ASD patients often
show their first signs and symptoms after multiple childhood immunizations.64
Rimland64 noted that the sharp rise in autism rates only
occurred after the multiple vaccine for measles, mumps, and rubella
(MMR) came into widespread use. In the US, children typically receive
as many as 33 vaccines before they can enroll in school, a dramatic
increase in the use of childhood vaccines over the last few decades.
Such vaccines often contain mercury and other preservatives.65
Commercial vaccines have been examined for contaminating microorganisms,
and one study found that approximately six percent of commercial
vaccines were contaminated with Mycoplasmas.69 Thus we
examined the extent of intracellular bacterial infections in patients
with ASD. We were aided in this examination by data that we collected
on families of Gulf War veterans where there was a documented, deployment-associated
Mycoplasma fermentans infection and a high incidence of autism in
their children after the infected veteran returned to the home.70
Previously, we found that veterans of the Gulf War with chronic
fatiguing illness (GWI) exhibited multiple nonspecific signs and
symptoms.71,72 Upon examination, the signs and symptoms
of GWI were indistinguishable from civilian patients diagnosed with
Chronic Fatigue Syndrome/Myalgic Encephalomyopathy (CFS/ME),71,72
except for symptomatic children aged three to 12 who were also diagnosed
with autism or attention deficit hyperactivity disorder (ADHD),
two disorders that fall under ASD.73 In our study, 45
of 110 GWI patients or ~42% had Mycoplasmal infections (Figure
3), and almost all of these (37 out of 45 or ~82%) were single
infections (one species of Mycoplasma).73 M. fermentans
was found in ~85% of these single infection cases (Figure
3). When the few multiple infection cases were examined,
most were found to have combinations of M. fermentans plus either
M. pneumoniae, M. hominis, or M. genitalium. In contrast, in healthy
control subjects only six of 70 subjects (8.5%) were positive for
any Mycoplasmal infection, and all of these were single infections
of various types.70,73 Comparing GWI patients and non-symptomatic
control subjects, there was a significant difference in the incidence
of Mycoplasmal infections (P<0.001). However, differences in
infection incidence or species of Mycoplasmal infection between
male and female GWI patients or male and female control subjects
were not seen. 70,73,74
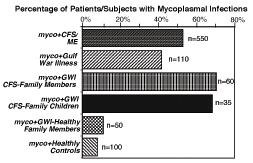 Figure
3: Percentage incidence of Mycoplasmal
infections in family members of verterans with Gulf War Illnesses.
The results were determined by PCR.70 Figure
3: Percentage incidence of Mycoplasmal
infections in family members of verterans with Gulf War Illnesses.
The results were determined by PCR.70
In family members of Gulf War veterans
with GWI, there was evidence of illness and Mycoplasma transmission.
We found that 57/107 (53.2%) of these family members from families
with one or more Gulf War veteran diagnosed with GWI and with a
positive test for a Mycoplasmal infection showed symptoms of CFS/ME.
Among the CFS-symptomatic family members, most (40/57 or 70.2%)
had Mycoplasmal infections compared to the few non-symptomatic family
members who had similar infections (6/50 or 12%) (Figure
3). When the incidence of Mycoplasmal infection was compared
within families, the CFS/ME family members were more likely to have
Mycoplasmal infections compared to non-symptomatic family members
(P<0.001).70 Symptomatic children (mostly diagnosed
with autism and ADD) were also infected with the same species of
Mycoplasma at high incidence (usually M. fermentans), and this was
not seen in aged-matched control subjects. Although some non-symptomatic
family members did have Mycoplasmal infections (5/50 or 10.0%),
this was not significantly different from the incidence of Mycoplasmal
infections in healthy control subjects (6/70 or 8.5%) (Figure
3).70
The Mycoplasma species was also similar between GWI patients and
their CFS/ME-symptomatic family members. In 45 Mycoplasma-positive,
CFS/ME-symptomatic family members, most (31 out of 40 or 77.5%)
had single species infections (almost all M. fermentans), similar
to the Mycoplasma-positive Gulf War veterans (37 out of 45 or 82%).
These results were highly significant (P<0.001). We did not find
differences in the incidence of infection or type of infections
between males and females, children versus adults, or spouses versus
other family members.70 However, similar to previous
reports, the time of onset of CFS/ME illness after the Gulf War
tended to be shorter in spouses than other family members, but these
differences did not achieve significance.
We next examined a small cohort of ASD patients in Central California.73
This comprised 28 patients aged three to 12 who were diagnosed with
ASD. Many of these children had at least one parent with a chronic
illness, and the most common diagnosis of adults or adolescents
in the same family was CFS/ME or fibromyalgia syndrome. When the
ASD patients were examined for Mycoplasmal infections, 15 children
tested positive (54%) for Mycoplasmal infections. However, in contrast
to the children of GWI patients, who for the most part had only
one type of Mycoplasmal infection, M. fermentans, the Central California
group tested positive for a variety of Mycoplasma species. We also
tested a few siblings without apparent signs and symptoms, and for
the most part, few had these infections (5/41 subjects or 12%).73
Similar results were found in the Gulf War veterans' families where
12% of non-symptomatic family members had Mycoplasmal infections.70
In another study, we examined the blood of 48 ASD patients from
Central and Southern California and found that a large subset (28/48
or 58.3%) of patients showed evidence of Mycoplasma spp. infections
compared to two of 45 (4.7%) age-matched control subjects (Odds
Ratio=13.8, P<0.001).75 Since ASD patients had a high
prevalence of one or more Mycoplasma species and some also showed
evidence of infections with Chlamydia pneumoniae, we examined ASD
patients for other infections (Figure 4).
In addition, the presence of one or more systemic infections may
have predisposed ASD patients to other infections, thus we examined
the prevalence of C. pneumoniae (4/48 or 8.3% positive, Odds Ratio=5.6,
P<0.01) and HHV-6 (14/48 or 29.2%, Odds Ratio=4.5, P<0.01)
co-infections in ASD patients. We found that Mycoplasma-positive
and –negative ASD patients had similar percentages of C. pneumoniae
and HHV-6 infections, suggesting that such infections occur independently
in ASD patients. Control subjects also had low rates of C. pneumoniae
(1/48 or 2.1%) and HHV-6 (4/48 or 8.3%) infections, and there were
no multiple infections in control subjects. The results indicated
that a large subset of ASD patients show evidence of bacterial and/or
viral infections (Odds Ratio=16.5, P<0.001).75
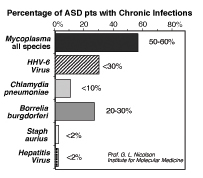 Figure
4: Percentage incidence of bacterial
and viral infections in 48 patients with Autistic Spectrum Disorders.75
The range indicates results from laboratories. Incidence was determined
by Western blot, serology, or PCR. Figure
4: Percentage incidence of bacterial
and viral infections in 48 patients with Autistic Spectrum Disorders.75
The range indicates results from laboratories. Incidence was determined
by Western blot, serology, or PCR.
Chronic Fatigue
Syndrome
Chronic fatigue syndrome (CFS/ME) is reported by 20% of all patients
seeking medical care.76 It is associated with many well-known
medical conditions and may be an important secondary condition in
several chronic illnesses. Although chronic fatigue is associated
with many illnesses, CFS/ME and fibromyalgia syndrome (FMS) are
distinguishable as separate syndromes based on established clinical
criteria.77 However, their clinical signs and symptoms
strongly overlap. CFS/ME is characterized by unexplained, persistent,
long-term, disabling fatigue, plus additional signs and symptoms,
whereas patients with FMS additionally suffer from muscle pain,
tenderness, and soreness.78 In patients with either diagnosis,
other conditions that can explain their signs and symptoms are absent;
thus in many patients with overlapping signs and symptoms, it is
difficult to make a clear distinction between a diagnosis of CFS/ME
and FMS.
Most CFS/ME and FMS patients have immunological abnormalities and
infections.67,68 Thus CFS/ME patients can be subdivided
into clinically relevant subcategories that may represent different
disease states or co-morbid conditions or illnesses.79
An important subset of CFS/ME patients is characterized by the presence
of chronic bacterial and viral infections.10,11,66-68
Identifying systemic infections in CFS/ME patients, such as those
produced by Mycoplasma species, Chlamydia pneumoniae, Brucella species,
Borrelia burgdorferi, and HHV-6 infections (Figure
5), is likely to be important in determining the treatment
strategies for these CFS/ME patients.11,79-81
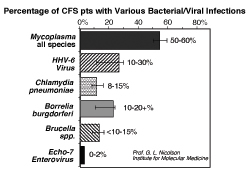 Figure
5: The incidence of various bacterial
and viral co-infections in 100 patients with CFS/ME. The bars indicate
the range of values found in different independent studies. Incidence
determined by Western blot or PCR tests of blood. Figure
5: The incidence of various bacterial
and viral co-infections in 100 patients with CFS/ME. The bars indicate
the range of values found in different independent studies. Incidence
determined by Western blot or PCR tests of blood.
Using the blood of 100 CFS/ME patients
and forensic polymerase chain reaction, we found that a majority
of patients show evidence of multiple, systemic bacterial and viral
infections (Odds Ratio = 18.0, 95% CL 8.5-37.9, P< 0.001) that
could play an important role in CFS/ME morbidity.11,79
CFS/ME patients had a high prevalence of one of four Mycoplasma
species (Odds Ratio = 13.8, 95% CL 5.8-32.9, P<0.001) and often
showed evidence of co-infections with different Mycoplasma species,
Chlamydia pneumoniae (Odds Ratio = 8.6, 95% CL 1.0-71.1, P<0.01),
and/or active HHV-6 (Odds Ratio = 4.5, 95% CL 2.0-10.2, P<0.001).
We found that eight percent of the CFS patients showed evidence
of C. pneumoniae and 31% of active HHV-6 infections.11,79
In a separate study, we found that a sizable percentage of CFS/ME
patients were infected with Borrelia burgdorferi, and therefore,
they were also Lyme disease patients.80
Lyme Disease
Lyme disease (LD) is the most common tick-borne disease in North
America. First described in Southeastern Connecticut in 1975, the
infection is caused by a tick bite and the entry of the spiral-shaped
spirochete Borrelia burgdorferi and other co-infections.82
Borrelia b. and its co-infections have been carried into new habitats
by a variety of ticks and their vectors. After incubation for a
few days to a month, the Borrelia spirochete and co-infections migrate
through the subcutaneous tissues into the lymph and blood where
they can travel to near and distant host sites.83 Transplacental
transmission of Borrelia b. and co-infections can occur in pregnant
animals, including humans, and blood-borne transmission in humans
by blood transfusion is likely but unproven. The tick-borne LD co-infections
can and usually do appear clinically at the same time.
Since the signs and symptoms of LD overlap with other chronic conditions,
LD patients are often diagnosed with other illnesses, such as CFS/ME
or rheumatoid arthritis. However, many patients with LD fail to
receive an adequate diagnosis for years, and during this period,
ineffective treatments may contribute to the refractory nature of
the disease.
About one-third of LD cases start with the appearance of a round,
red, bulls-eye skin rash (erythema migrans) at the site of the tick
bite, usually within three to 30 days.83 Within days
to weeks, mild flu-like symptoms can occur that include shaking
chills, intermittent fevers, and local lymph node swelling. After
this localized phase, which can last weeks to months, the infection(s)
can spread to other sites (disseminated disease), and patients then
show malaise, fatigue, fever and chills, headaches, stiff neck,
facial nerve palsies (Bell's palsy), and muscle and joint pain,
and other signs/symptoms.83
LD can eventually become persistent or chronic and involve the central
and peripheral nervous systems as well as ophthalmic, cardiac, musculoskeletal,
and internal organ invasion. At this late chronic stage, rheumatoid
arthritis, neurological impairment with memory and cognitive loss,
cardiac problems (mycocarditis, endocarditis causing palpitations,
pain, bradycardia, etc.), and severe chronic fatigue are often apparent.84,85
The late chronic phase of the disease usually overlaps with other
chronic conditions, such as CFS/ME, FMS, rheumatoid arthritis, among
others, causing confusion in the diagnosis and treatment of the
chronic phase in LD patients.80.85 Some contend that
this late phase is not even related to LD, resulting in failure
to successfully identify and treat the chronic condition.86
The involvement of co-infections in causing chronic signs/symptoms
in LD patients has not been carefully investigated; however, such
infections on their own have been shown to produce comparable signs/symptoms.
Diagnostic laboratory testing for LD at various clinical stages
is, unfortunately, not full-proof, and experts often use a checklist
of signs and symptoms and potential exposures, along with multiple
laboratory tests to diagnose LD.86 The laboratory tests
used for LD diagnosis include the following: detection of Borrelia
b. surface antigens by enzyme-linked immunoassay (EIA), immunofluorescent
assay (IFA), and Western immunoblot of Borrelia proteins.86
Alternatively, polymerase chain reaction (PCR) for Borrelia DNA
has been used to detect the DNA of the intact organism in blood.85
A true-positive test result usually consists of more than one positive
test from the above list, often EIA followed by Western immunoblot.87
The problem with these tests is that they are blood tests that require
the presence of antibodies or Borrelia proteins in the blood, or
they are dependent on the spirochete and thus its DNA being present
in the blood (PCR).
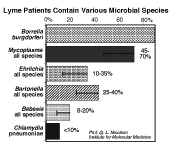 Figure
6: The incidence of various bacterial
co-infections in 100 patients with Lyme disease. The bars indicate
the range of values found in various laboratories. Incidence determined
by seriology, Western blot and PCR tests of blood. Figure
6: The incidence of various bacterial
co-infections in 100 patients with Lyme disease. The bars indicate
the range of values found in various laboratories. Incidence determined
by seriology, Western blot and PCR tests of blood.
We80,85 and others88
have found that the most common co-infection found with Borrelia
b. are various species of Mycoplasma (Figure
6). Approximately 50-70% of LD patients also have Mycoplasmal
co-infections (M. fermentans > M. pneumoniae, M. hominis >
M. penetrans, other species). In some cases, multiple Mycoplasmal
infections are present in LD patients.80 The presence
of Mycoplasmal infections complicates the diagnosis and treatment
of LD, and some of the generalized signs/symptoms found in Borrelia-positive
patients are also found in Mycoplasma-positive patients. Like the
Borrelia b. spirochete, Mycoplasma species are found at intracellular
locations in various tissues and are only rarely found free in the
blood. This can make detection difficult, and, in some patients,
the appearance of Borrelia b. and various Mycoplasmas in their white
blood cells can be cyclic.
Other LD co-infections include Ehrlichia species, Bartonella species,
and Babesia species.89 Ehrlichia species are small, gram-negative,
pleomorphic, obligate intracellular infections similar to mycoplasmas
in their structures, intracellular locations, and resulting signs/symptoms.90
The other common bacterial co-infection is caused by Bartonella
spp.,91 and this co-infection (along with Mycoplasma
spp.) appears to be one of the most common tick-borne co-infections
found with Borrelia burgdorferi.91 Bartonella spp., such
as Bartonella henselae, which also causes cat-scratch disease,92
is often found in neurological cases of Lyme disease.91
A non-bacterial co-infection found with Borrelia burgdorferi is
the intracellular protozoan Babesia species.93 There
are over 100 species of the genus Babesia, but most Lyme disease
co-infections in humans in North America are caused by Babesia microti.94
About 10-40% of cases of LD show Babesia co-infections (Figure
6).
The combination of Borrelia, Mycoplasma, and Babesia infections
can be lethal in some patients (about seven percent of patients
can have disseminated intravascular coagulation, acute respiratory
distress syndrome, and heart failure), but the majority of LD patients
with Babesia spp. have the chronic form of the infection. These
patients can show mild to severe hemolytic anemia (probably correlating
with the protozoan colonization of erythrocytes, which can be seen
by experienced individuals in blood smears) and a normal to slightly
depressed leukocyte count. However, this is usually not seen in
patients who have progressed to the chronic phase of the disease.93
The chronic form of LD with CNS invasion is usually called neuroborreliosis,
and this can be a fatal disease.84
Final Comment
Chronic illness patients are at risk for a variety of opportunistic
infections, including bacterial, viral, and fungal infections. These
can complicate diagnosis and treatment, and they may be a particular
problem in the late, chronic phase of the disease. Late-stage patients
with neurological manifestations, meningitis, encephalitis, peripheral
neuropathy, or other signs and symptoms may have complicated co-infections
that are not recognized or treated by their physicians.
The neurological signs and symptoms in many – more likely
most – chronic illness patients are usually due to systemic
chronic infections that penetrate the CNS. Such infections often
follow acute or chronic heavy metal, chemical, biological (viral,
bacterial, fungal infections) exposures or other environmental insults
or even multiple vaccines that have the potential to suppress the
immune system and allow opportunistic infections to take hold. These
illnesses generally evolve slowly over time in a multi-step process
that likely requires genetic susceptibility along with multiple
toxic exposures. Because of this, they are particularly difficult
to treat using single modality approaches. Importantly, if complex,
chronic infections are ignored or left untreated in these illnesses,
it is unlikely that recovery will follow. We have also stressed
that integrative approaches to therapy offer the most realistic
chance for patients to eventually recover.
An integrative approach to the treatment of the complex, slow-growing
intracellular infections found in a variety of chronic diseases
requires long-term treatment with antibiotics and other antimicrobials,
and it also requires dietary supplementation to restore normal homeostasis.85,94
For example, most if not all chronic illness patients require dietary
supplementation with vitamins, minerals, amino acids, lipids, and
other natural supplements.85,94-97 These are necessary to restore
intracellular functions that are damaged by infections and also
damaged by environmental stresses, heavy metals, chemicals, and
other contaminating substances. Improving or restoring normal neurological,
immunological, and hormonal functions to patients with complex neurodegenerative
and other chronic diseases remain difficult and important goals.

The Institute for Molecular Medicine
16371 Gothard Street H
Huntington Beach, California 92647
949-715-5978; Fax: 714 596-3791;
gnicolson@immed.org
www.immed.org
Page 1, Notes
|



![]()
![]()
![]()

 Figure
1:
Figure
1:  Figure
2:
Figure
2:  Figure
3:
Figure
3: Figure
4:
Figure
4: Figure
5:
Figure
5:  Figure
6:
Figure
6: 