By Petra Davelaar Dorfsman, ND
Thorough understanding of the causation of pathological processes is fundamental to clinical care as it informs and guides the approach to treatment.
In this article, I will first discuss the prevailing cancer-causing paradigms upon which our current oncology standard-of-care is largely based. When I say paradigms, I specifically mean the two main narratives that have been the foundation of cancer treatment by most medical centers and academic institutions around the world. These have given rise to a huge industrial complex involving government agencies, pharmaceutical and biomedical firms, hospitals and clinics, universities, professional societies, nonprofit foundations, and the media.1
The two paradigms are 1) Cells start proliferating in an uncontrolled fashion as a result of tissue damage; 2) The genetic theory of cancer, also known as the somatic mutation theory.
I will also cover other theories and conjectures of the cause of cancer, describe discoveries made in cancer metabolism research, and touch on the hallmarks of cancer.
Then I will discuss submolecular regulation. In fact, it is submolecular dysregulation due to deuterium accumulation that causes cancer. Submolecular regulation is the underlying mechanism foundational to the architecture of all of the various prevailing theories. Submolecular dysregulation is indeed what gives rise to uncontrolled proliferation, genome transformation, the process of rapidly dividing cells into new phenotypes–what is being described as evolution—and metabolic dysfunction.
Three decades of basic research have confirmed the importance of deuterium in living organisms. The ratio of the two stable hydrogen isotopes (hydrogen/deuterium) is a key signal influencing cellular processes at the submolecular level. The transfer of hydrogen and deuterium to different structural and functional molecules is essential for maintaining normal cellular functions, nucleic acid structure, and protein integrity.
Deuterium availability regulates cell growth and is “crucial to the start of cell proliferation” by modulating DNA structure and size.2
To provide context for the significance of this isotope ratio, the concentration of deuterium in our body is two- to four-times higher than the normal amount of circulating blood glucose. Deuterium is present five times higher than calcium and 10 to 18 times that of magnesium.
When the concentration increases to levels above a relatively stable equilibrium, exceeding our physiological and biochemical capacity for deuterium, submolecular dysregulation is the result. Depletion can reverse dysregulation as has been demonstrated in the published research. Downstream effects of submolecular dysregulation are many and vary greatly. It is those effects that have been pulling research scientists in all directions. Many contradict one another.
Integral to submolecular regulation is the ability to efficiently fractionate or discriminate deuterium. Excess deuterium in mitochondria and the cytoplasm is what drives submolecular dysregulation, leading to cancer. This knowledge has largely been ignored.
Paradigm One
Cancer is a disease of excessive growth. Cancer begins when cells acquire the ability to grow uncontrollably and ultimately invade and damage the body’s normal tissues. Cancer development happens in multiple stages, from precancerous changes to malignant tumors. This can be a result of an injury, a toxin, smoking, radiation, chemicals, UV light, viruses, etc.
The treatment approach is to remove and/or kill the abnormal cells with surgery, chemotherapy, and radiotherapy.
Paradigm Two
In 1914, Theodor Boveri published On the Origin of Malignant Tumors,3 widely acknowledged to be the seminal publication in cancer pathogenesis. Boveri proposed two theories:
- Proliferation is the default state of cells.
- Cancer is a cell-based disease.
He concluded that cancer is a problem of cell proliferation and that cancers are due to an abnormal chromosomal rearrangement that eliminates a portion of chromosomal material whose function it is to inhibit cell proliferation. This was a theory; the actual structure of DNA had not been discovered yet.
Shortly after Boveri’s death in 1915, RC Whitman introduced the notion that the cancer cell was a “mutated” cell and coined the idea of a somatic mutation to explain what Boveri had implied in 1914. This is when the somatic mutation theory was born.
The premise that cancer is a cell-based disease in which acquired DNA mutations affect the control of cell proliferation remains the dominating dogma today; it continues to be put forth as authoritative while lacking substantial grounds. As it has been deemed as an unquestionable fact, it has determined the direction of cancer research and treatment approach for the last 50 years.
There are indeed familial, or inherited, cancers but they are relatively rare, accounting for approximately 5 to 10% of all cancers. And given the right conditions, maintaining mitochondrial deuterium depleting function, these cancers will likely not develop.
With the sequencing of the human genome, we expected to get all the answers. In fact, we got very few. In 2022 the final 8% was completed. The project cost 2.7 billion US dollars and was funded primarily by the US Department of Energy, the NIH, and the UK’s Medical Research Council and Wellcome Trust.4,5
Turns out just a few forms of cancer can be attributed to a single mutation, for example, 80% of chronic myeloid leukemia is caused by a single chromosomal translocation of genetic material between chromosomes 9 and 22 that is thought to occur spontaneously.
The number of mutations found in any cancer can vary from a few (2–8) to many more, usually hundreds or thousands. In metastases in the same patient and in different parts of the tumor, we will find completely different mutations.
Memorial Sloan Kettering conducted analyses of 25,000 patients over a period of six years and explored “the question of whether it’s possible to look at an individual tumor and, based on its genomic profile alone, predict its precise future metastatic trajectory, the answer is clearly no.”
The study was revealing in other ways: the data showed that metastatic disease is genomically entirely different from the primary disease. They analyzed over 50 tumor types and close to 100,000 metastases.6
A 2019 PLOS paper7 takes a different approach to finding out why someone develops cancer; the researchers designed what they call the multi-combination multi-hit model of carcinogenesis. They concluded that there is no single combination of mutations responsible for all instances of cancer, even within a cancer subtype, and that carcinogenesis is a result of one of many possible combinations of somatic mutations. So instead, they count the number of mutations for a given cancer.
Another important clue of the failure of the somatic mutation theory is the fact that cancer patients have lots of mutations, but so do cancer-free people. A 2012 detailed analysis of 31,717 cancer cases concluded that “the vast majority of, if not all, aberrations that were observed in the cancer-affected cohort were also seen in the cancer-free subjects.”8
A clear example of the debatable relevance of mutation profiles in cancer comes from a systematic review from the Institute of Inflammation and Ageing at University of Birmingham. They demonstrate that “of the 17,371 human genes appearing in at least one paper in PubMed, 15,233 or 87.7% also appeared in at least one paper mentioning cancer. This impressive result tells us that, if practically any gene is associated with cancer, which is the most probable case, looking at single gene explanations is devoid of any heuristic power.”9,10
The reality is that there are millions of mutations. The Sanger Institute’s COSMIC group is responsible for the “Catalogue of Somatic Mutations in Cancer.” They released their most recent version earlier this year.11
The Pan-Cancer Analysis of Whole Genomes Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), characterized mutational signatures using 84,729,690 somatic mutations from 4,645 whole-genome and 19,184 exome sequences that encompass most types of cancer.12
84+ million mutations.
Then there are those, who after a cancer diagnosis undergo extensive genetic analysis, and we find no genetic mutations at all. This Genome Wide Association studies of cancer shows that 66 to 80% of breast, colon, and prostate cancer cannot be explained by genetics.13
Deuterium Is an Oncoisotope
How we can “explain” these findings is that the 84+ million different mutations, epigenetics and non-mutational cancers are due to genome transformation as a result of deuterium accumulation. This is why we call deuterium an oncoisotope.14,15,16,17
DNA synthesis takes place in all cells through the process of DNA replication and is changed by deuteration. The kinetic isotope properties of deuterium will affect the RNA/DNA shape, function, and size. Nucleotide and histone modifications can be altered by deuterated methyl groups. The increased bonding strength affects the efficiency of DNA methyl transferases and DNA repair enzymes. If insertion of a deuteron into the DNA backbone happens more frequently, the cell will end up with additional DNA in the cytoplasm or nucleus or both; this is called aneuploidy.
A normal human somatic cell wraps all newly synthesized DNA in 46 chromosomes before dividing. If there is too much DNA, which is termed as chromosomal abnormalities, the cell will produce additional chromosomes to wrap it all up neatly.18 Again, this is aneuploidy or cancer. For example, a pancreatic cancer cell (MIA PaCa) will have 52 chromosomes, a metastatic lung cancer cell 61 (H441). This is due to excess deuterium present in the cell.11
In fact, 90% of tumors have gained or lost chromosomes. Copy number changes far exceed the proportion affected by sequence changes. Deuterium can increase copy number, structural chromosome abnormalities, and contribute to genome instability (CIN).19
DNA strand breaking by hydroxyl radicals depends on the accessibility of specific bonds on the DNA backbone. In 1998 original data from a team of the Department of Chemistry, among others, at Boston University, published the effects of deuteration of specific carbons on DNA. The 5th and 4th carbon are most sensitive to deuteration and the increase in strength required to break such a bond is 2.6 to 2.1 times.20
In 2014 they showed similar data on deuteration of RNA; a similar increase in strength is required to break these bonds.21,22
The treatment approach for this second paradigm has been to develop targeted gene therapies, immunotherapies such as monoclonal antibodies and CAR-T therapies, signaling pathway inhibitors such as cyclin-dependent kinase (CDK) 4/6 inhibitors, BcrAbl tyrosine-kinase inhibitors, PD-1 and PD-L1 checkpoint inhibitors, CTLA-4 inhibitors, PI3K inhibitors, CRISPR and Yamanaka factors, and the already failed Sleeping Beauty transposon system.
Magic or Wandering Bullets
The Journal of the American Medical Association published in 2020, a comparative effectiveness study of 92 novel cancer therapies approved for 100 indications over 17 years. They concluded that the therapies were associated with substantial tumor responses but prolonged median overall survival by only two-to-four months.23 Unfortunately, this increase in median overall survival time is negligible, especially compared with deuterium depletion.24,25,26
“We hand these drugs out like water but have no idea how they function in the body. We only know how they behave in rodents. It is like throwing spaghetti on the wall and see what sticks,”said Thomas Marron, MD, PhD, Assistant Director for Early Phase and Immunotherapy Trials at the Tisch Cancer Institute in a 2021 presentation. 27
Some research scientists already knew this in 2005: “Although new drugs are continuously designed and tried, it seems inevitable that genetic and signal protein targets pose too broad flexibility and variability, often changing target characteristics and thus escape treatments turning ‘magic bullets’ into rather ‘wandering bullets.’”28
The reality is that many of these drugs have very serious, sometimes fatal, effects.29,30,31
How does this continue to go on you may ask? Regulatory capture of our three-letter government agencies plays a direct role in the ongoing marketing efforts. A drug’s review and approval process are often expedited while based on incomplete data sets as pharmaceutical industry-funded patient advocacy organizations (PAOs) demand and receive faster drug approval and easier access.32
Another factor is that randomized clinical trials in oncology have evolved since its widespread adoption in the 1970s. Contemporary oncology randomized clinical trials have significantly changed and now largely measure by assumed surrogate end points and are almost exclusively funded by the pharmaceutical industry.33 “Assumed surrogate end point” means that the trials no longer report on overall survival time or quality of life improvement of the patients, which is what patients ultimately care about: real results.
Somatic Mutations Are Not the Cause
“The clinical reality is that it is not single genes, but rather the properties of aneuploid-based methylated networks that allow metastatic cancer cells to explore novel niches in different genetic backgrounds and to rapidly become resistant to drug-based therapies,” states George Gabor Miklos.34 He is correct. This is due to the accumulation of deuterium affecting those methyl groups.
There have been numerous papers published over the last several decades that have attempted to refute, dispute, and take down the somatic mutation theory as clinical data show little support for the theory measured against patient outcome data.35,36,37
Before I get to submolecular dysregulation caused by deuterium accumulation, I just want to discuss a few other theories.
The Evolutionary Theory of Cancer
This bioinformational crisis in cancer research led to the blossoming of the evolutionary theory of cancer.
What Dr. Jason Fung calls Paradigm 3.0 in his latest book The Cancer Code builds on both the evolutionary theory and the atavistic theory.38
He writes: “Cancer is, improbably, a backward of evolution, or atavism, toward the single-cell organism from our evolutionary past.” His book is a paradox to contend with. Fung does an excellent job describing how the genetic paradigm has failed and then makes a case for how these same mutations by evolutionary force through Darwinian selection and selection pressure cause cancer.
Atavistic is derived from atavus, meaning ancestor. The atavistic model posits that cancer cells have reverted to doing things that our cells used to do a long time ago–– namely, survive in the absence of oxygen. It postulates that cancer initiation and progression represent reversions to ancestral unicellular phenotypes.
The missing piece in their theory is that when mitochondria fail within a cell due to deuterium, the cell loses function. It may seem that this is reverting back to a unicellular organism. But that is not the case.
Jason Fung was not the first, however. Peter Nowell,39 Mel Greaves40 (seen as the “father” of the evolutionary theory of cancer), and many others have published papers and books since 1976.
Their premise is that cancer is the result of clonal or somatic evolution, which is explained as “the process of sequential acquisition of vertically transmittable genetic/epigenetic elements in multicellular organisms.” Evolutionary forces are the driver. Selection pressure determines the heterogeneity of the tumor, meaning the diversity of the mutations.
It is hard to imagine that a self-terminal disease process has anything to do with the survival of the fittest. This whole new focus of cancer as an evolutionary process comes just as Darwin’s theory of evolution is shaking in its boots as new research is clearly invalidating his poetic take on how organisms evolve. The elaborate colorful evolutionary theory of cancer offers no solutions, just more mutations and confusion.
Theories Continued
There are many more theories: The trophoblast theory, the tissue organization field theory, the brand-new egg cell’s genetic program theory, the stem cell origin of cancer theory. (There are stem cells and there are stem cells that have accumulated deuterium driving them to divide.)
What unifies all the previously discussed theories is that deutenomics, the science of how water moves in our bodies, was not ever considered.
Our current treatment paradigm is obsolete; “a new paradigm should reflect that cancer is a systemic disease rather than an invading organism or mere cluster of mutated cells that need to be eradicated.”41
It is time for a common-sense revolution in oncology where we prioritize treatments that meaningfully improve survival and quality of life.42
Cancer Metabolism
Metabolism is integral to submolecular regulation.
That cancer is a submolecular process associated with electron transport abnormalities and protein dysfunction, was first introduced by Albert Szent-Györgyi in “The Living State and Cancer” in 1979.43
The understanding that cancer is a metabolic disease started when Otto Heinrich Warburg, almost 100 years ago, observed and published what is known as the Warburg effect.
Recent advances in metabolism allowed us to follow the precise metabolic flux or movement of precursors and substrates. Using tracer-based metabolomics, important mechanisms and pathways have been identified. Metabolic profiling involves labeling common precursors such as glucose, lactate, or acetate, with an isotope so that the pathway this molecule travels can be traced. They use mass spectrometry and stable isotopes such as carbon 13 or deuterium to observe and trace metabolic pathways for gluconeogenesis, glycogen synthesis, lipid synthesis, pentose- and TCA cycle metabolism, among others.44
As a result, nonoxidative pentose phosphate pathways and their direct role in ribose synthesis in tumors was established as the cause of cancer based on the model of cellular glucose metabolism in 1998.45
In 2002, metabolic profiling elucidated the mechanism of the antiproliferative action of Imatinib, also known as Gleevec. Rather than an unknown signaling pathway inhibition mechanism that was claimed, Imatinib controls flux of metabolites reversing the metabolic transformation observed in the chronic myeloid leukemia cells. Imatinib limits glucose-metabolizing enzymes by improving mitochondrial functions via enhanced fatty acid oxidation and metabolic water production.46
Hallmarks of Cancer
A well-known series of papers that have attempted to create “an organizing principle for rationalizing the complexities of neoplastic disease,” in the authors’ own words, are the hallmarks of cancer review papers. There are now three in the series, and they have over one-hundred thousand citations all together. The first paper was published in 2000.47
The second update entitled “Hallmarks of Cancer: New Dimensions” was published in 2022. The 2000 paper introduces their six core hallmarks. Both the 201148 and the 202249 have added four emerging and enabling characteristics each.
By definition a hallmark is a defining feature, a distinguishing characteristic that is part of every phenotype related cellular behavior but none other. A single feature. Not 14.
Additionally, the ‘hallmark’ features contradict one another. In 2022 Hanahan states that genome or DNA instability and mutation is a fundamental component of cancer formation and pathogenesis. Then introduces an emerging hallmark called nonmutational epigenetic programming describing mutation-less cancer.
The 2011 paper added as an emerging hallmark: deregulating cellular energetics, also referred to as reprogramming energy metabolism.
“Altered energy metabolism is proving to be as widespread in cancer cells as many of the other cancer-associated traits that have been accepted as hallmarks of cancer. This realization raises the question of whether deregulating cellular energy metabolism is therefore a core hallmark of cancer cells that is as fundamental as the six well established core hallmarks.”
In fact, deregulating cellular energetics is THE hallmark of cancer. They confirm that with the 2022 New Dimensions update. This is submolecular dysregulation.
I’m not alone in making this statement, many others have. In a 2022 paper “Cancer as a metabolic disorder,” the authors state that “according to the metabolic theory of cancer, the somatic mutations and all other hallmarks of cancer are downstream epiphenomenon occurring from the initial disturbances of cellular energy metabolism.”50
Furthermore, in their 2011 “Hallmarks of Cancer: The Next Generation,” the authors stated on page 15: “A functional rationale for the glycolytic switch in cancer cells has been elusive….” Actually…we did know this.
That same year in 2011, original data published in Cancer Cell clearly implicated a deficiency of the mitochondrial metabolic enzyme fumarate hydratase as the cause of the glycolytic shift. They also demonstrated how restoration of the enzyme activity reversed glycolytic and tumorigenic features of the fumarate hydratase deficient cells.51
A 2014 PLOS publication further details how fumarate hydratase-deficient kidney cancer cells undergo metabolic remodeling, with changes in mitochondrial respiration, glucose, and glutamine metabolism.52 These changes represent multiple biochemical adaptations in glucose and fatty acid metabolism that support malignant proliferation.
The authors again demonstrated that the restoration of fumarate hydratase function leads towards a normal metabolic phenotype, rather than a lactic fermentation driven “Warburg”-like phenotype, without additional changes. What this data tells us is that by restoring metabolic water recycling pathways we can reprogram and reverse the cancerous phenotype of transformed cells.
In 2016 another landmark paper was published that explained in detail how submolecular dysregulation leads to cancer entitled: “Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle.”53
This is the paper that identified the precise mechanisms by which our bodies keep deuterium away from the mitochondrial matrix. Years earlier Olgun described how deuterium damages the delicate ATP synthase nanomotors of mitochondria.54
The role of deuterium in biology is a potential missing piece in the elusive cancer puzzle,53 explaining cancer epidemics in western populations as it seemingly correlates with excessive deuterium loading from processed carbohydrate intake55 in place of natural fat consumption.56
These findings and the application of tracer-based metabolomics have provided new insight to the Warburg effect.
In the cancer process, glycolysis can no longer deplete deuterium because there is no deuterium depleted water available in the mitochondria and/or cytoplasm. Deuterium destroys proton tunneling, hydrogens stop moving via tunneling and they don’t deliver energy through nanomotor movements. Protons cannot get into the matrix and hydrogens pile up outside the mitochondria still residing on lactate.
This is the Warburg effect, the single hallmark of cancer. These are not theories. These are facts.
Submolecular Dysregulation
Mitochondrial health determines cellular health, not genetics. Thomas Seyfried in his 2012 book Cancer as a Metabolic Disease57 details nuclear cytoplasmic transfer experiments that started as early as 1982. Essentially what all experiments did was they placed a nucleus of a cancer cell in the cytoplasm of a healthy cell to see if it would become cancerous. It did not. They also placed a nucleus of a healthy cell in a cancer cell to see if it would restore the cell, it did not. The nucleus contains the genetic material, which contains all the alleged mutations. These experiments demonstrated conclusively that the somatic mutation theory is incorrect.
The health of mitochondria depends on its ability to produce deuterium-depleted metabolic water and the efficiency of the TCA cycle to keep deuterium out of the mitochondrial matrix.
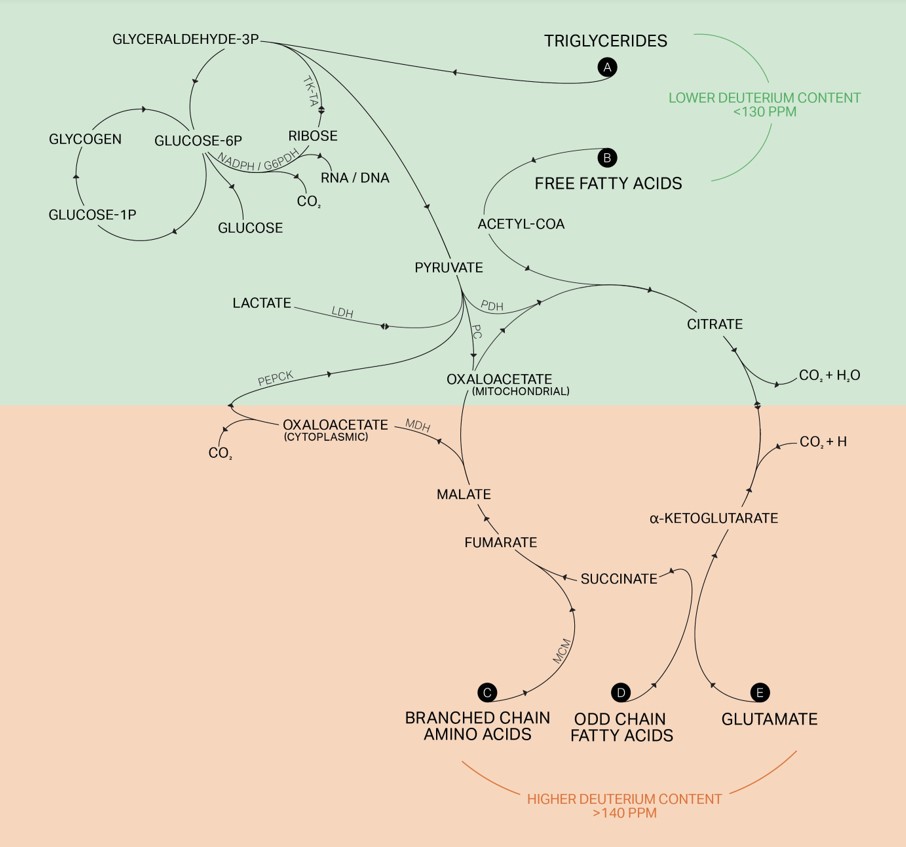
The TCA cycle is the chemical conversion of carbohydrates, fats, and proteins into metabolic water and CO2, which also generates energy in the form of ATP and heat. Glycolysis is the pathway employed by all tissues for the breakdown of glucose to provide energy and intermediates for other metabolic pathways, including the TCA cycle. The phenotype is the observable characteristics or traits of an organism. i.e healthy cell or a cancerous cell.
Phenotype and adaption depend on mitochondrial metabolism. It is the health of mitochondria that determines the health of the cell and its fate. If the deupleting (deuterium-depleting) functions of mitochondria are defective, or overburdened by any number or combination of factors, submolecular dysregulation and cancer are the result.
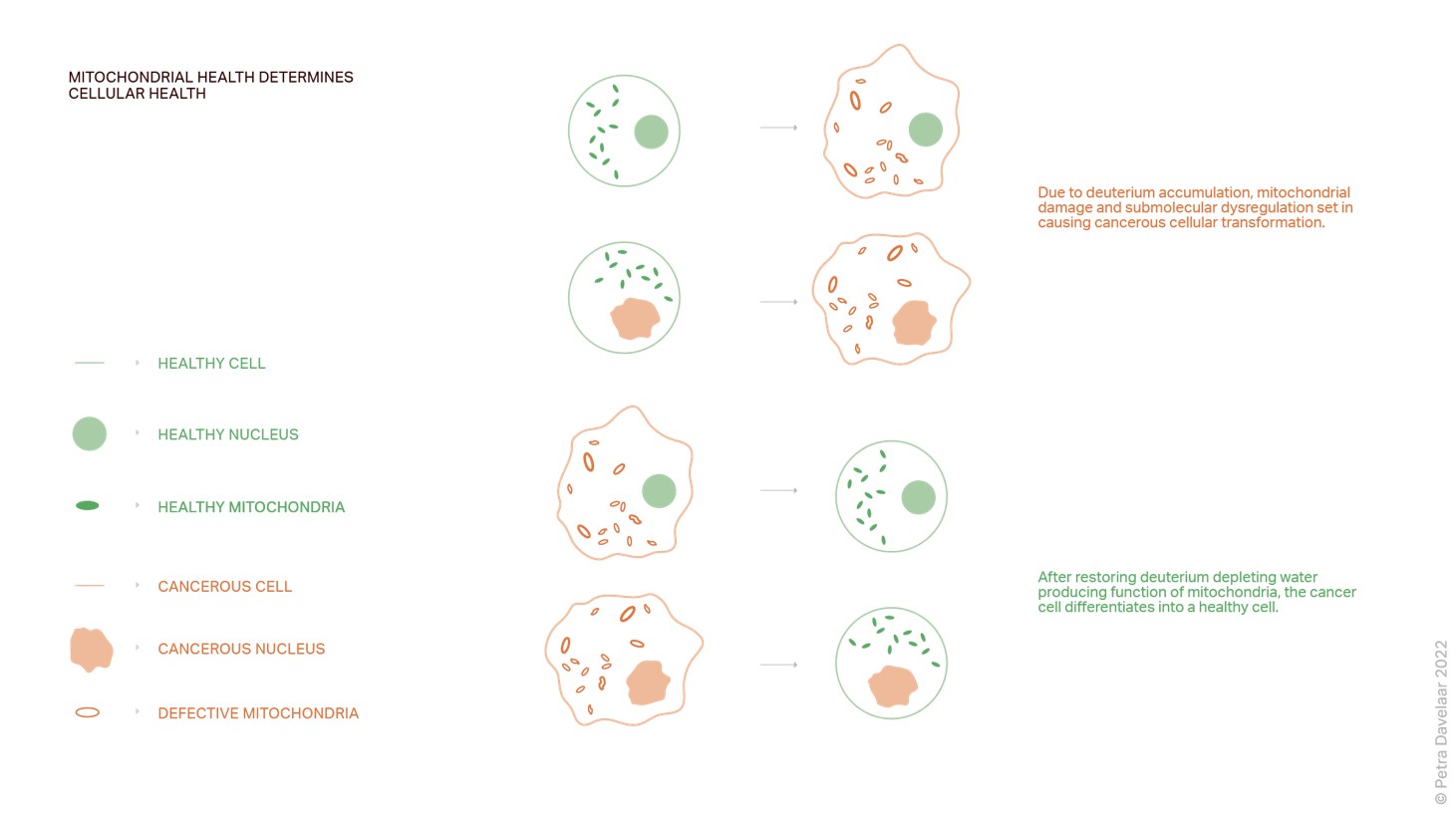
This figure illustrates beautifully how, due to deuterium accumulation, mitochondria become enlarged, misshapen, and lose their cristae. This is when submolecular dysregulation sets in causing cancerous transformation, regardless of the status of the nucleus. After restoring deuterium-depleting water producing and recycling functions of the cancer cell, it differentiates into a healthy cell.
The fumarate hydratase data informed us that if we restore the metabolic water recycling function of the enzyme, we restore dysfunctional mitochondria and reverse the cancerous transformation process.
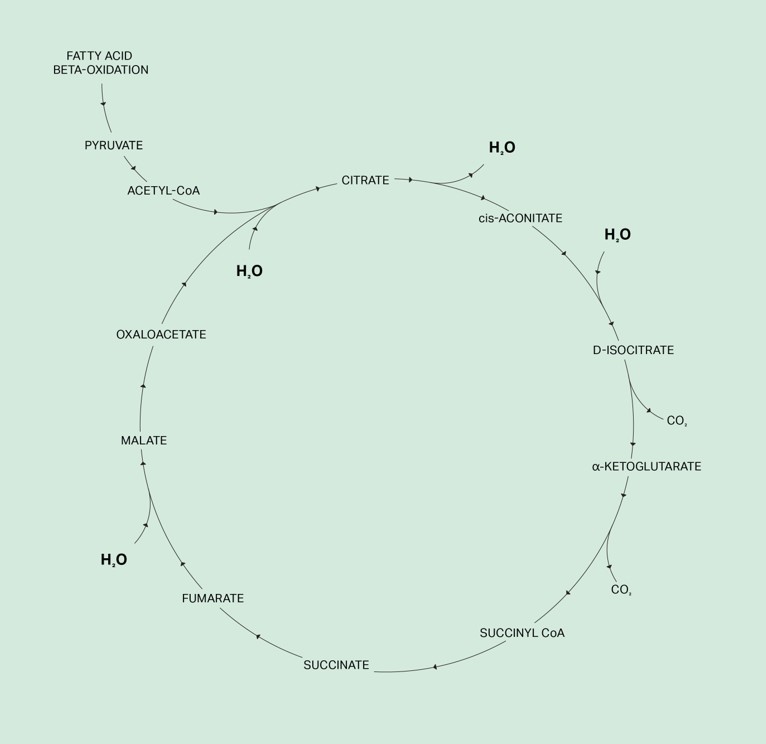
What is the function of fumarate hydratase you may ask? There are 8 enzymatic reactions in the TCA cycle. Three of these reactions add deuterium-free water to TCA cycle intermediates from water that is produced in the mitochondrial matrix, also known as metabolic water. Citrate synthase is the first of the three enzymes involved in these water reactions as it adds one water molecule to form citrate from oxaloacetate and acetyl CoA.
The second enzyme is aconitate hydratase or aconitase. This is a two-step reversible reaction and involves dehydration followed by hydration. This is a water exchange reaction; one water molecule is removed and replaced with a deuterium-depleted water molecule from the matrix.58
The third enzyme involved in adding water is fumarate hydratase, which catalyzes the reversible hydration of fumarate to malate. If fumarate hydratase is deficient, submolecular dysregulation sets in causing cancerous transformation.
To understand the following electron microscope images and figures of the 2011 Glycolytic shift paper I mentioned earlier, let’s define a few terms:
- HLRCC stands for hereditary leiomyomatosis renal cell carcinoma.
- UOK262 is the name of an immortalized cell line. UOK262 cells are derived from a patient who had aggressive hereditary leiomyomatosis-associated recurring kidney cancer.
- UOK262WT is the cell line restored to a wild type with normal fumarate hydrates deupleting functions, which means the normal healthy phenotype.
- 117C4 + 786OWT = non-cancerous renal cells, or healthy kidney cells
- 3-NPA is an inhibitor of the TCA cycle enzyme succinate dehydrogenase.
The arrows point to the dysfunctional mitochondria.
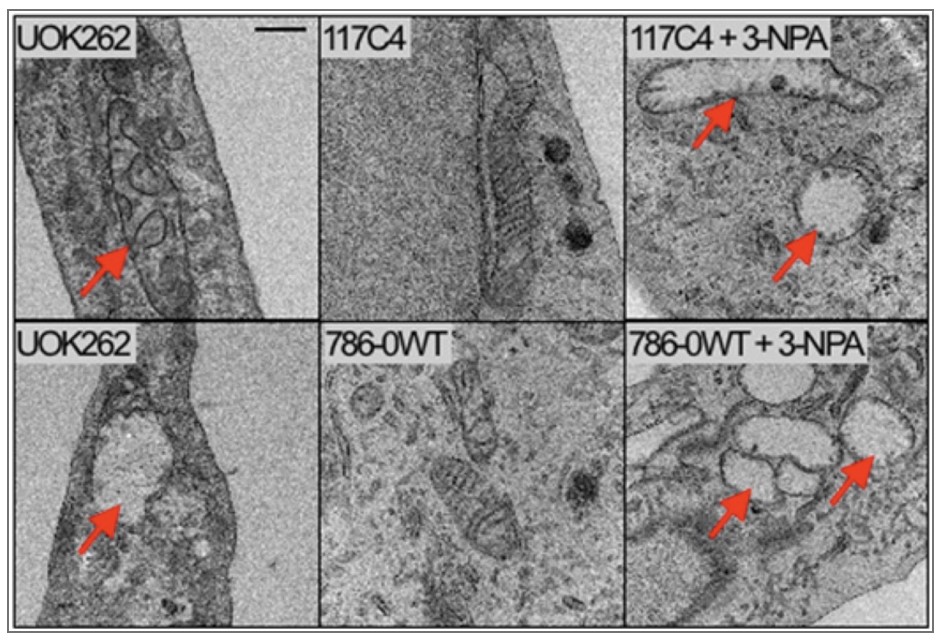
You can see swollen mitochondria with severe fragmentation of the cristae in the cancer cells on the 2 left photos similar to abnormalities found in non-cancerous renal cells that were treated with the succinate dehydrogenase inhibitor. This is another mitochondrial enzyme as well as Complex II of the electron transport chain. The middle images are of healthy untreated cells and show smaller mitochondria where the cristae are visible. These are functional mitochondria.
These experiments demonstrate submolecular dysregulation. Healthy cells that lose deupleting function transform into cancer cells.
This next graph, from the same paper, took it one step further. To examine tumorigenicity of the cancer cells, the UOK262 cells were injected subcutaneously into nude mice. Palpable tumors developed within 32 days, with a tumor doubling time of ∼17 days. Animals injected with UOK262-Wild type did not develop tumors indicating that fumarate hydratase dysfunction was required for tumor growth.
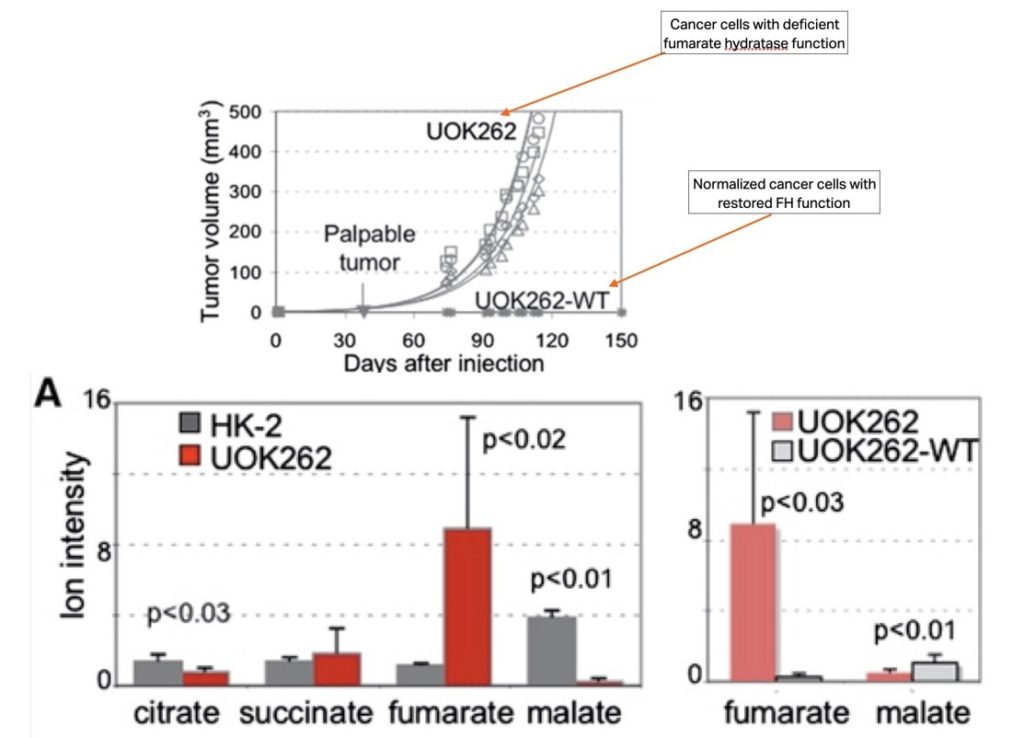
This graph shows a typical organic acids test. The gray bars are the normal healthy kidney cells and the red the cancer cell line. Notice how in fumarate hydratase deficient cancer cells fumarate piles up, while no malate is produced. On the right, the data shows that in the grey ‘normalized ‘cancer cell, the wild type in which fumarate hydratase function is restored, fumarate is readily catalyzed into malate.
This is a real-life organic acids test result from a patient with a succinate dehydrogenase deficiency due to homozygous variation of two of the four succinate dehydrogenase genes. This allosterically affects the other enzymes leading to submolecular dysregulation and tumor formation. This causes the same dysfunctional mitochondria as I showed on the previous slide, the normal cells that were treated with 3-NPA, a succinate dehydrogenase inhibitor.
Genetic mutations only matter if they compromise deuterium-depleting functions.
The experiments I discussed can be described as the fumarate hydratase mutation model. Thankfully, these mutations are rare.
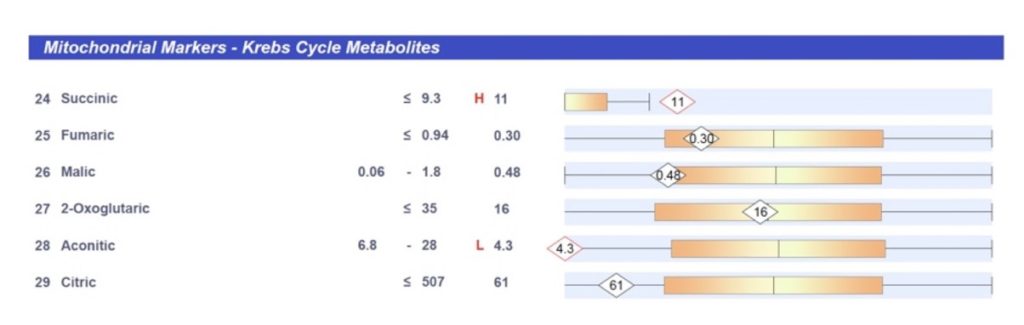
What is not rare and the cause of most mitochondrial dysfunction is the lack of oxygen due to old age, COPD, damage to or glycosylation of circulating red blood cells, underlying metabolic diseases like diabetes, obesity and deuterium overloaded mitochondria in all kinds of cells in any tissue, and many other factors which I will discuss next.
The lack of oxygen directly affects the amount of deuterium depleted metabolic water produced in mitochondria.
FOOD + Oxygen (O2) = Metabolic deupleted water H2O + CO2 + ATP + Heat
The 2014 PLOS metabolic programming study also determined the differences in oxygenation rate and extracellular acidification between the UOK262 cancer cells and the UOK262 Wild Type cells. The wild type cells had a four-fold higher oxygenation rate and two-fold lower acidification rate.
This is significant. Oxygen consumption directly relates to the amount of deuterium depleted water produced in mitochondria and the extracellular acidification relates to the water piling up, known as hydropic degeneration, this is metabolic crowding. In the fumarate hydratase model, which is rare, they’ve demonstrated that after we restore the metabolic water recycling function of the enzyme, we restore dysfunctional mitochondria and reverse the cancerous transformation process.
In clinical care, these genetic therapies cannot accomplish this. It is not possible to repair genes in all somatic cells as we cannot switch genes around in every cell. What can compensate for dysfunctional mitochondrial enzymes and restore mitochondrial enzymes and mimic mitochondrial function is to deplete deuterium in all the ways we currently know how.
Restoring Submolecular Dysregulation
We approach deuterium depletion from all possible pathways. Let’s start with the fuel we provide our bodies.
First and foremost, it is the quality and kind of food you consume. Focus on those that are low in deuterium. This means a seasonal and local low-carbohydrate well-raised meat-based or ketogenic-type diet that matches your heritage and preferences. Choose the best available sources for all food you consume; fats should be as unprocessed as possible, proteins ideally come from animals that were grown slowly and respectfully in their preferred habitat and fed the best possible diet. Vegetables should be pesticide- and herbicide-free.
Include fats that come from grass-fed butter, lard, tallow, suet, duck fat, coconut, and olive oil. Avoid industrial fats such as soy, canola, corn, cottonseed, safflower, sunflower, and grapeseed.
The importance of the quality of the food cannot be overstated. Grass-fed meats and dairy are much lower in deuterium content compared to meat and dairy from grain-fed animals, as shown in this 2022 Metabolomics paper.59
Deuterium Accumulation
The flow chart above shows what key factors tend to accumulate deuterium levels in our bodies and the effects that they have. Water quality and quantity, fat quality and quantity, and light spectra are the primary inputs that determine deuterium levels.
Omnipresent glyphosate, electromagnetic frequencies, inorganic metals and trace minerals all affect submolecular regulation. Deuterium levels that exceed the physiological threshold leads to metabolic crowding, genome transformation,
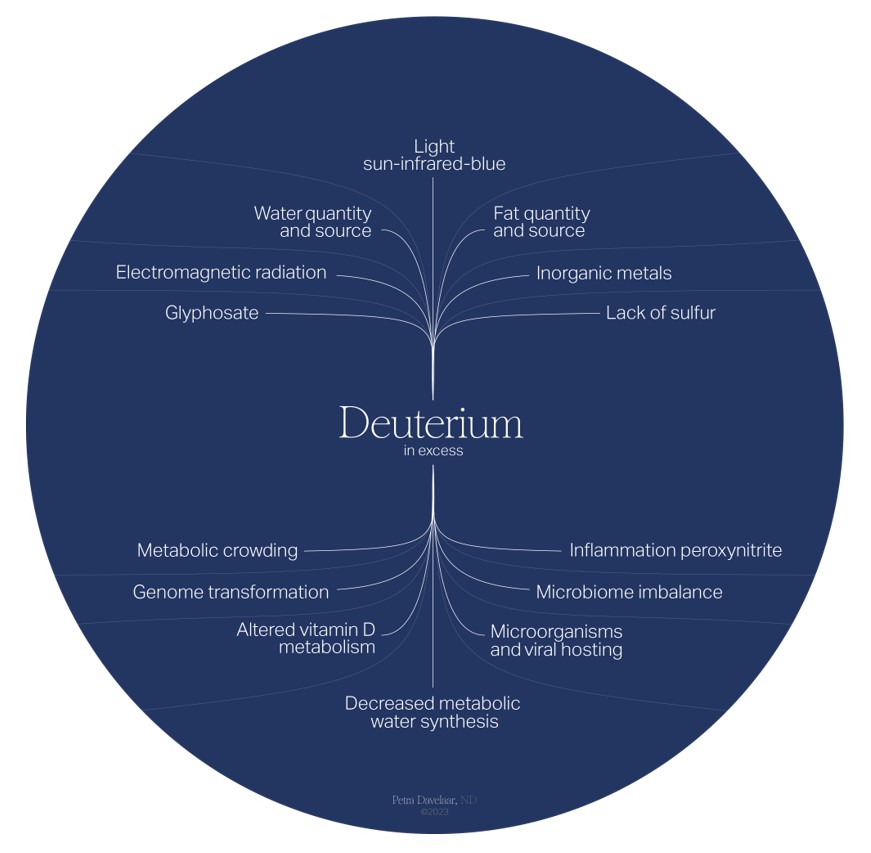
microbiome imbalance, viral and microbial hosting (deuterium is a growth factor for these organisms), inflammation, altered vitamin D and A metabolism, and, ultimately, decreased deuterium-depleted metabolic water synthesis.
Submolecular dysregulation due to the accumulation of deuterium is at the heart of the cellular transformation causing cancer. Approaching management and treatment of cancer based on submolecular dysregulation by targeting metabolic pathways in every way that we know today offers clear strategies to the management and prevention of cancer.
Deutenomics is revealing new mechanisms by which we can improve our cellular energy metabolism, protein integrity in order to reverse submolecular dysregulation every day.
To facilitate cellular reprogramming and reverse submolecular dysregulation, which will increase metabolic water production and bring the body in coherence, we have created a success list of aspects of daily life to focus on and integrate one by one where needed and possible.
- Consider your water, the deuterium content, the quality and quantity
- Consider fasting, both intermittent and with clear liquids
- Consider the light, your light environment of all spectra and intensities, including photodynamic therapy, photonbiomodulation, sunlight, UV light, and infrared light
- Adherence to circadian rhythms
- Include movement, acupuncture, and massage
- Consider temperature flux, including cold thermogenesis and sauna therapies
- Electromagnetic frequencies mitigation
- Optimize sleep environment
- Try different breathing techniques and realize that oxygen is one of the two rate-limiting factors of metabolic water production, besides protons.
- Add deuterium-scavenging botanicals if needed
Lastly, there is no question that deuterium-depleted water can reverse submolecular dysregulation very rapidly and efficiently. It can be used as wellness water or as a therapy, meaning the ppm you consume is based on optimal physiological levels and determined based on weight, diagnoses, consumption, etc.
For those who have been diagnosed with a cancerous process, adding deuterium depleted water to other therapies has shown to increase overall survival time three- to five-fold.24,25,26
Benefits of deupletion with deuterium-depleted water include the following:
- Inhibits cell proliferation24,60
- Prevents the D/H ratio from increasing to the threshold required for cell division61
- Induces cell cycle arrest62
- Induces apoptosis63,64,65
- Increases autophagy66
- Can selectively stress tumor cells and not healthy tissue67
- A decrease in tumor growth and metastasis rate66
- Restores activity of SOD66
- Restores mitochondrial fumarate hydratase activity51
- Prevents the expression of cancer-related genes, tumor development, and tumor recurrence61
- Reduces the number of single-stranded DNA breaks and modifies the miRNA profile66
- Decreases expression of p53, c-myc, and Ha-ras genes68
- Depletion protects cells by maintaining strong hydrogen bond networks53
- Is radioprotective69
- Improves thermoregulation70
- Is anti-inflammatory71,72
- Gives rise to oxidative stress to which cancer cells are particularly vulnerable60
Peer-reviewed data for the use of deuterium-depleted water in cancer patients:
- Deuterium depletion inhibits cell proliferation, RNA and nuclear membrane turnover to enhance survival in pancreatic cancer 24
- Deuterium depletion may delay the progression of prostate cancer25
- A retrospective study of survival in breast cancer patients undergoing deuterium depletion in addition to conventional therapies26
- A retrospective evaluation of the effects of deuterium depleted water consumption on four patients with brain metastases from lung cancer.73
As a true detective at heart, Petra integrates the most current scientific understanding of metabolism into her personalized approach to preventing and reversing disease.

Dr. Petra is a naturopathic doctor who practices Deutenomics, which is the science of how water moves in the body. She was born and raised in the Netherlands and moved to New York in her 20s. She has spent the last ten years in California where she graduated from Bastyr University in 2016. After four years of seeing patients in Santa Monica, she currently only consults patients via telemedicine. Since January 2021, her credentials have also been recognized and certified in Hungary, which extends to most other European countries.
She is known for her in-depth research and detailed follow through on each and every one of her patients. Her passion for seeking truth guides her to understand how we can do better in restoring patients’ health.

